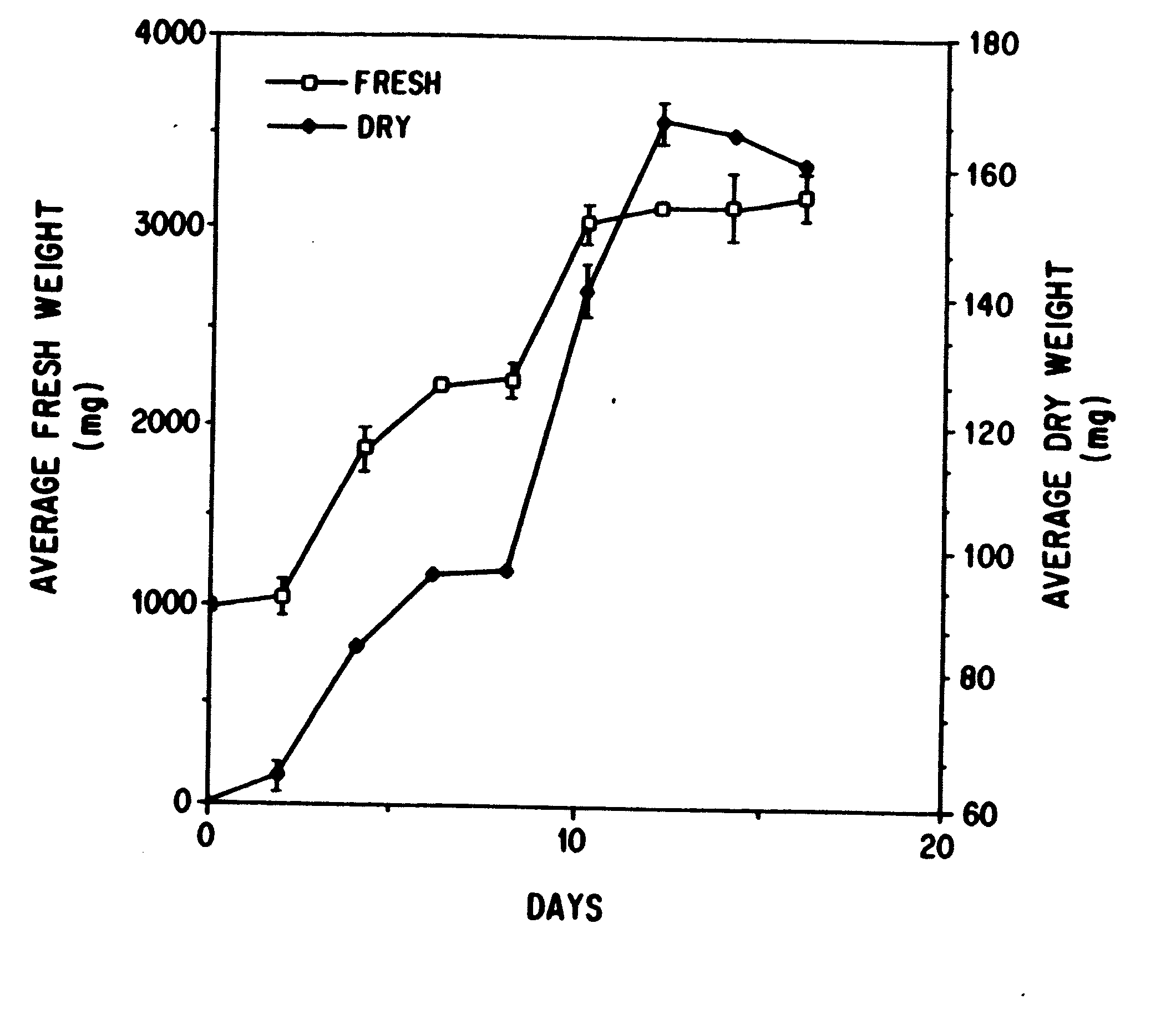
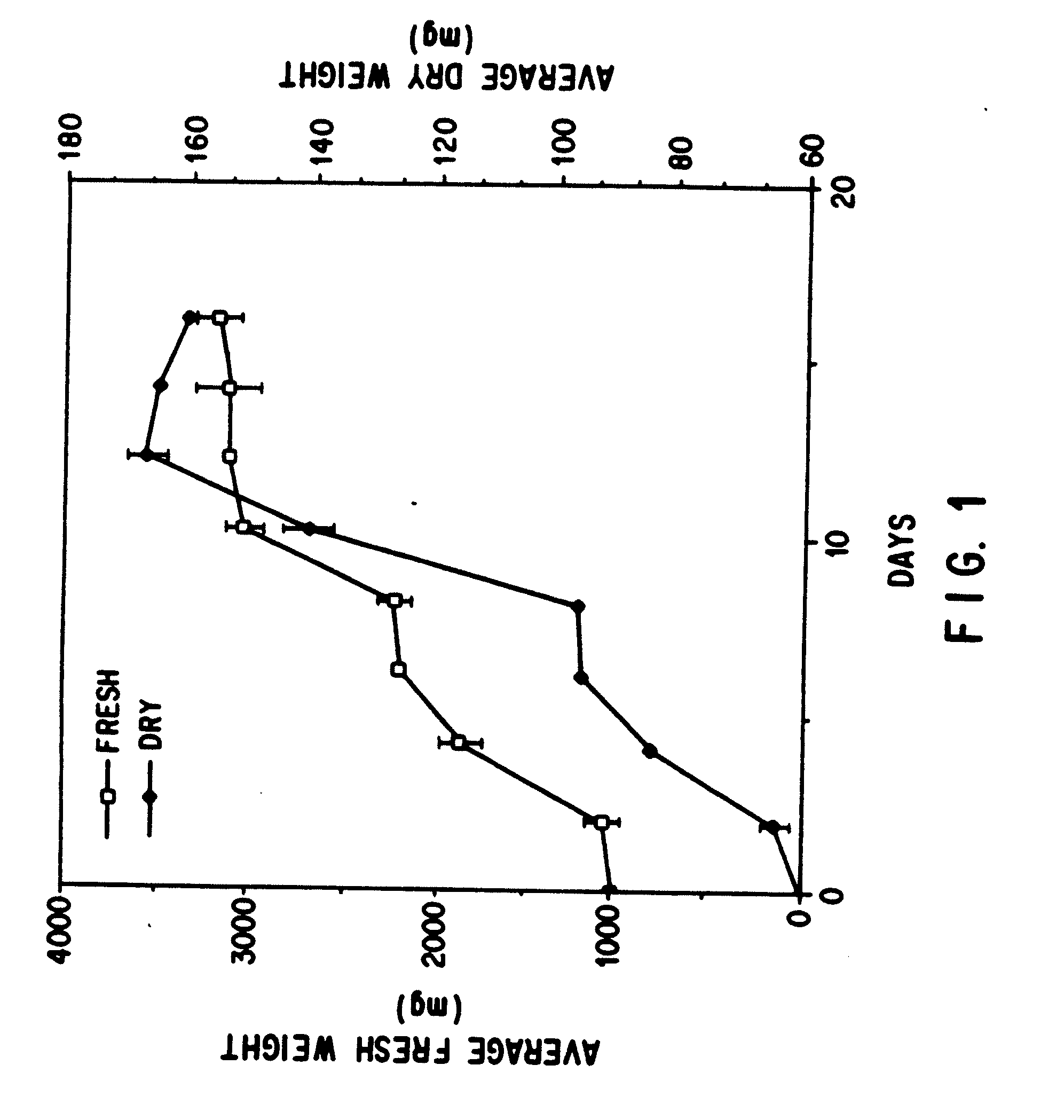
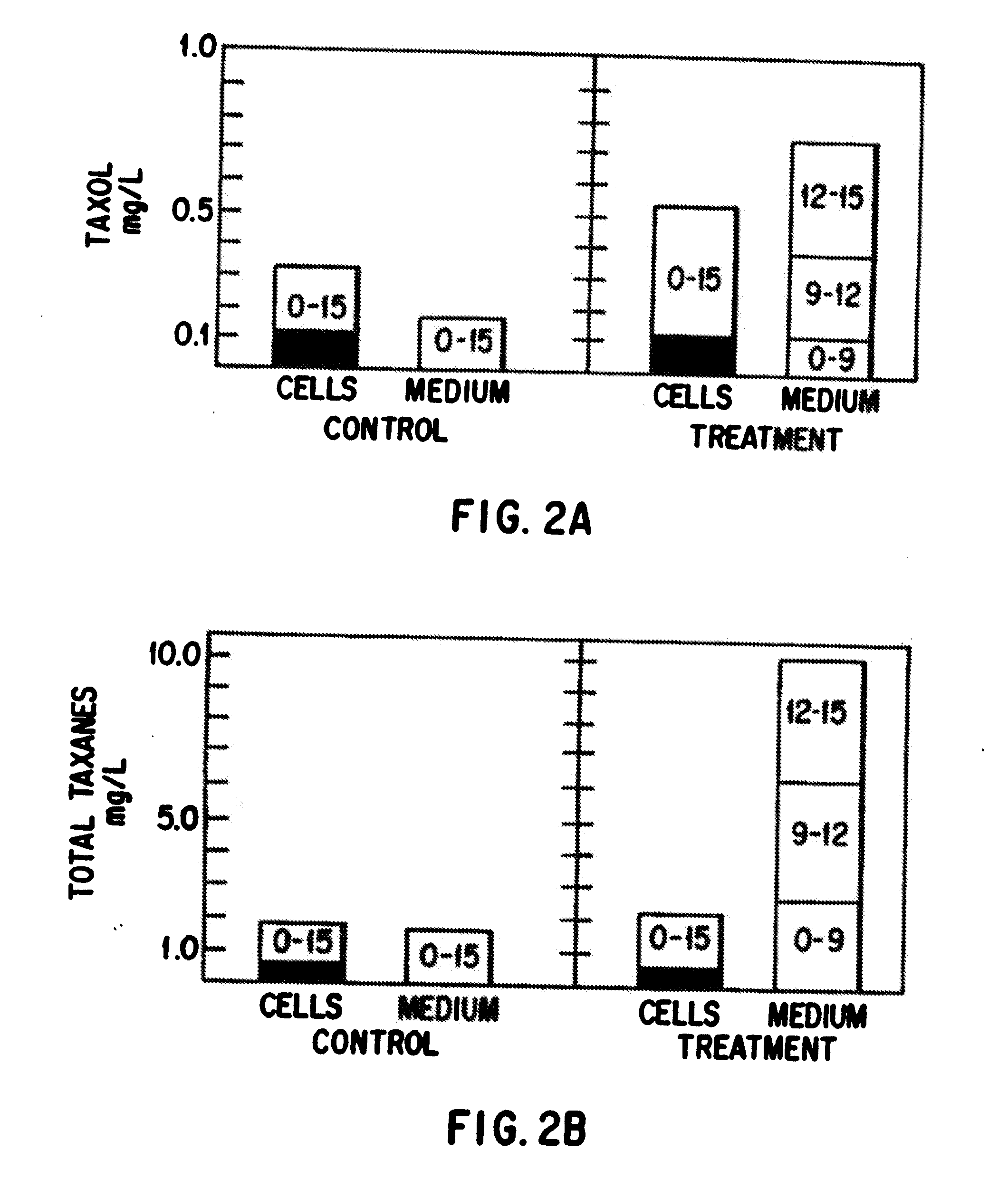
Enhanced production of taxol and taxanes by cell cultures of taxus species
a cell culture and taxanomy technology, applied in the field of enhanced production of taxol and taxanomy, can solve the problems of low production efficiency of taxonomy, difficulty in inducing rapid biosynthesis compared to herbaceous species, and low production efficiency of total synthesis, so as to achieve high cell viabilities, high cell densities, and rapid growth
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0114] Samples of Taxus plant material were collected from a number of wild and cultivated plants. Samples were processed upon arrival at the laboratory or stored at 4° C. until they could be used.
[0115] The material was first washed in dilute soap solution, rinsed in water, and the surface sterilized in a CLOROX solution (1% hypochlorite, pH 7) for 10 minutes. Under sterile conditions the material was then rinsed 3 times with sterile water. Needles were then cut in a 1% polyvinylpyrrolidone (PVP) solution with 100 mg / l ascorbic acid. Needles were placed with the cut end in Medium E (see Table 2). Thirty to forty explants were cultured per plate of medium. Plates containing explants were incubated at 25±1° C. in the dark. Plates were monitored daily for the appearance of contaminating micro-organisms, and where they were present, uncontaminated needles were removed and placed in a fresh plate of Medium E. Substantial callus formation was observed and the callus wa...
example 2
Callus Proliferation
[0116] Once calli were removed from the explant, they were cultivated at 25±1° C. in the dark. Healthy parts of the callus were transferred to fresh medium every 7 to 10 days, and this frequency of transfer was found to be extremely important for prevention of browning and for prolonged callus maintenance. The preferred growth and maintenance media for calli of various species are summarized in Table 3.
example 3
Suspension Initiation
[0117] 1 g fresh weight of callus material was aseptically inoculated into a 125 ml Erlenmeyer flask containing 25 ml of liquid medium appropriate to each species (see Table 3). For example, Medium D was used for Taxus chinensis. The flask was covered with a silicone foam cap (Bellco, N.J.) and placed on a gyratory shaker at 120 rpm at 24±1° C. in darkness. Suspension cultures were formed in approximately 3 to 10 days. Initially, medium was exchanged by suction filtering the flask contents through a buchner funnel containing a miracloth filter (Calbiochem), and resuspending all the biomass in fresh medium. Upon cell growth, 1-2 g (fresh weight) of cells, and were generally transferred into a new 125 ml flask containing 25 mL of fresh medium and were thereafter subcultured weekly.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com