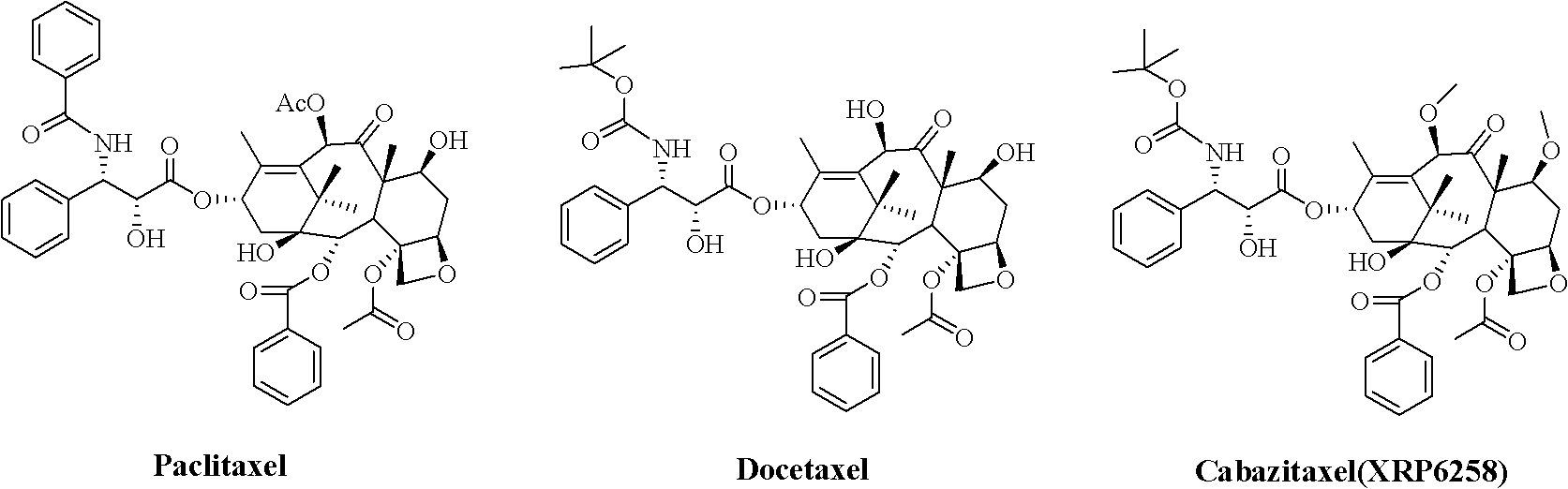
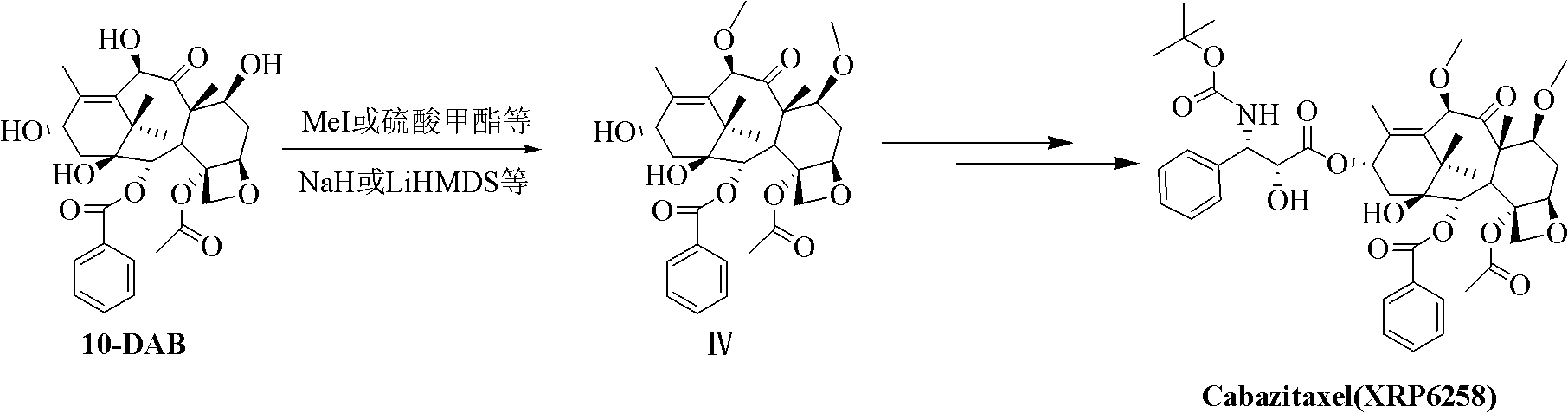
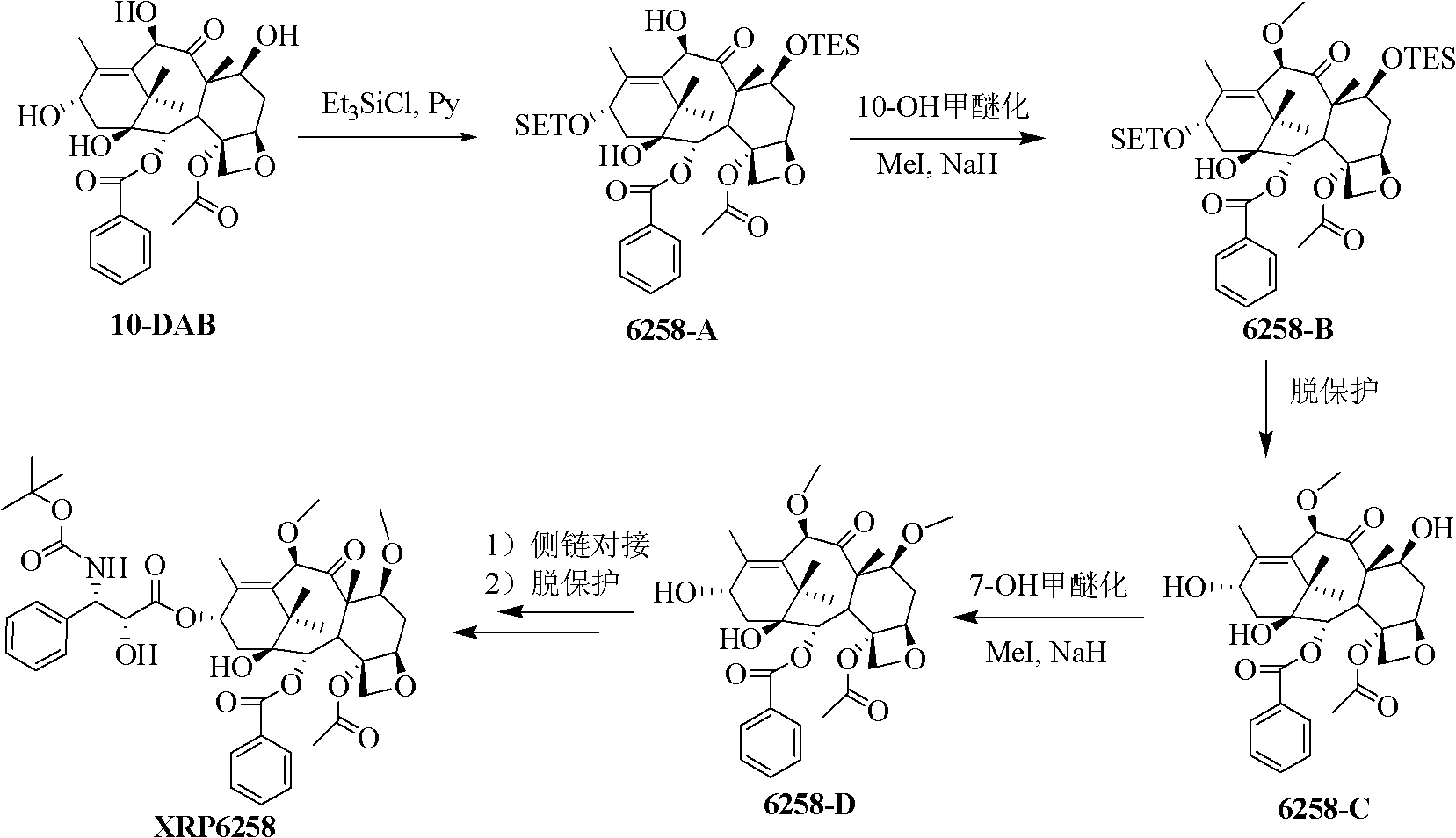
Preparation method of taxol anticancer drugs XRP6258
An anticancer drug, paclitaxel technology, applied in antitumor drugs, drug combinations, organic chemistry and other directions, can solve the problems of long route, difficult purification, long synthesis route, etc., and achieves simple preparation process, simple purification process, and easy operation. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] 1. Preparation of Compound II
[0052]
[0053] 10-DAB(I) (27.3g, 50.0mmol) was dissolved in dry DMSO (200mL), acetic anhydride (200mL) was added, protected by argon, stirred at room temperature overnight, and after the reaction was detected by thin layer chromatography, the reaction solution was Concentrate under reduced pressure and evaporate to dryness, dilute the resulting residue with ethyl acetate (1.5L), and wash with saturated sodium bicarbonate solution (300mL×6), distilled water (200mL×3), and saturated aqueous sodium chloride solution (200mL×3) successively , dried over anhydrous sodium sulfate, and concentrated to obtain light yellow foamy solid product II (28.1 g, yield about 85.1%), which was directly carried out to the next step without purification; 1 H-NMR (400MHz, CDCl 3 ): δ8.06(d, 2H, J=7.0Hz, Ph-H), 7.62(t, 1H, J=7.6Hz, Ph-H), 7.49(t, 2H, J=7.6Hz, Ph-H ), 5.80(s, 1H, H-10), 5.67(d, 1H, J=6.7Hz, H-2), 4.94(d, 1H, J=9.2Hz, H-5), 4.84(d, 1H , J=1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com