Preparation and application of taxane prodrug
A technology of taxanes and prodrugs, which is applied in the field of taxanes prodrugs and their preparation, can solve the problems of strong irritation, poor targeting, increased stability and targeting, and achieve good stability, Good tumor cell targeting effect and improved long cycle time effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Paclitaxel Prodrug Nanoparticles
[0043] Accurately weigh 10.0 mg of PTX-SS-CIT and 2.5 mg of poloxamer 188 and dissolve them in an organic solvent, slowly add them dropwise into 5 mL of buffer solution at room temperature, stir at 500 r / min for 1 h to allow the organic solvent to dry naturally, and obtain the final precursor drug nanoparticle solution.
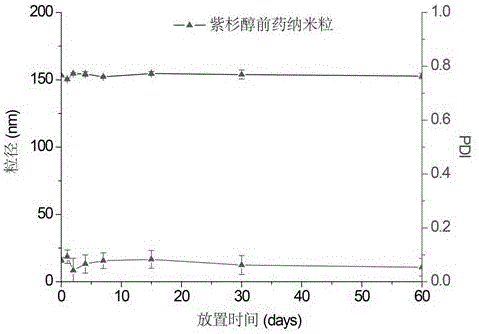
[0044] The paclitaxel prodrug nanoparticles of the present invention have better stability. The stability of nanoparticles was examined by room temperature placement experiment, and the particle size and PDI of nanoparticles were measured at 0 day, 1 day, 2 days, 4 days, 7 days, 15 days, 1 month, and 2 months. The results showed that paclitaxel prodrug nanoparticles had good stability, and no change in particle size occurred during placement. (See figure 1 )
Embodiment 2
[0046] Paclitaxel Prodrug Nanoparticles
[0047] Precisely weigh 10.0mg of PTX-SS-CIT and 1.0mg of Tween 80 and dissolve them in an organic solvent, slowly drop them into 5mL of buffer solution at room temperature, stir at 400r / min for 2h, and let the organic solvent dry up naturally to obtain the final prodrug Nanoparticle solution.
Embodiment 3
[0049] Cabazitaxel Prodrug Nanoparticles
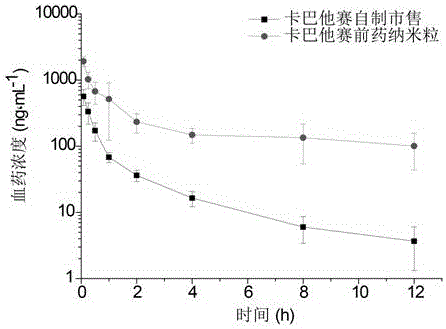
[0050] Accurately weigh 10.0 mg of CTX-SS-CIT, 2.0 mg of distearoylphosphatidylethanolamine-polyethylene glycol 2000 and dissolve them in an organic solvent, slowly add them dropwise into 5 mL of buffer solution at room temperature, stir at 800 r / min for 1 h to make the organic The solvent was evaporated to dryness naturally to obtain the final prodrug nanoparticle solution.
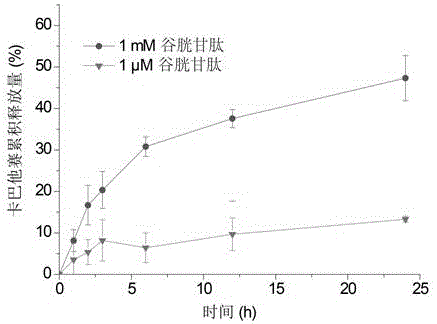
[0051] The cabazitaxel prodrug nanoparticles of the present invention have better redox-sensitive release behavior. Using pH 7.4 phosphate buffer (containing 0.5% Tween 80) with two glutathione concentrations (1mM or 1μM) as the release medium, take an appropriate amount of nanoparticles and place them in a dialysis bag at 37.0°C with a rotation speed of 100r / Under the condition of 1 min, take 1ml of the solution at 1h, 2h, 3h, 6h, 12h, and 24h respectively, filter through a 0.45 μm filter membrane, discard the initial filtrate, and use the subsequent filtrate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com