Compound, preparation method thereof and application of compound in preparation of cabazitaxel
A compound and reaction technology, which is applied in the preparation of chemical drugs and the field of pharmacy, can solve the problems of large amount of solvent usage, complex structure, and many side reactions, and achieve small impact on operators and the environment, simple post-processing, and low solvent usage little effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-1
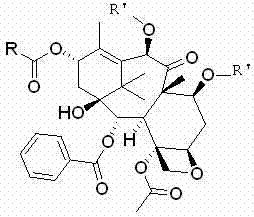
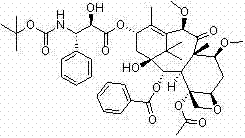
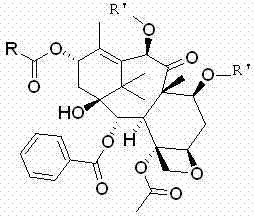
[0095] Example 1-1 7,10-di-O-(2,2,2-trichloroethoxycarbonyl)-13-O-acetyl-10-deacetylbaccatin III
[0096] First add 1L of anhydrous toluene to the reaction flask, and mix 100g (0.11mol ) into it, after the dissolution is complete, cool the internal temperature of the reaction solution to 0°C, add 62mL (0.44mol) of triethylamine, continue to maintain the internal temperature at 0°C, and slowly add 15.8mL (0.22mol) of acetyl chloride dropwise to control the reaction The inner temperature of the liquid does not exceed 10°C. After the dropwise addition, keep stirring and react for 0.5h, then raise the temperature to 80°C and keep warm for 24h. A large amount of solids are precipitated. It was determined by TLC that the reaction raw materials had been reacted, slowly poured into saturated aqueous sodium bicarbonate solution, added ethyl acetate to extract the aqueous phase, TLC spot plate confirmed that there was no product remaining in the aqueous phase, washed the organic phase w...
Embodiment 1-2
[0098]Example 1-2 7,10-di-O-(2,2,2-trichloroethoxycarbonyl)-13-O-benzoyl-10-deacetylbaccatin III
[0099] First add 500mL of anhydrous dimethylformamide to the reaction flask, and 50g (56mmol) of 7,10-bis-O-(2,2,2-trichloroethoxycarbonyl)-10-deacetylbaccatin III After the dissolution is complete, cool the internal temperature of the reaction solution to 0°C, first add 31mL (0.22mol) of triethylamine, and then slowly add 13.0mL (0.11mol) of benzoyl chloride dropwise, and control the internal temperature of the reaction solution not to exceed 10°C, after the dropwise addition, keep stirring and react for 0.5h, then raise the temperature to 95°C and keep warm for 24h, a large amount of solids are precipitated. After the reaction was completed, slowly pour it into saturated aqueous sodium bicarbonate solution, add ethyl acetate to extract the aqueous phase, wash the organic phase with water and saturated brine, and dry over anhydrous sodium sulfate. Filter, and evaporate the filt...
Embodiment 1-3
[0101] Example 1-3 7,10-di-O-(2,2,2-trichloroethoxycarbonyl)-13-O-p-methoxybenzoyl-10-deacetylbaccatin III
[0102] Add 350 mL of anhydrous toluene to the reaction flask, dissolve 35 g (39 mmol) of 7,10-bis-O-(2,2,2-trichloroethoxycarbonyl)-10-deacetylbaccatin III into it, and completely After dissolving, cool the internal temperature of the reaction solution to 0°C, first add 22mL (0.16mol) of triethylamine, then slowly add 10.6mL (78mmol) of p-methoxybenzoyl chloride, and control the internal temperature of the reaction solution to not exceed 10°C After the dropwise addition, the reaction was carried out with heat preservation and stirring for 0.5h, and then the temperature was raised to 100°C for 24h with heat preservation, and a large amount of solids were precipitated. After the reaction was completed, slowly pour it into saturated aqueous sodium bicarbonate solution, add ethyl acetate to extract the aqueous phase, wash the organic phase with water and saturated brine, an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com