Preparation method of taxanes compound
A compound, the technology of docetaxel, which is applied in the field of preparation of taxane compounds, can solve the problems of many reaction steps and long reaction time, and achieve the effect of less reaction steps, short preparation time and improved purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
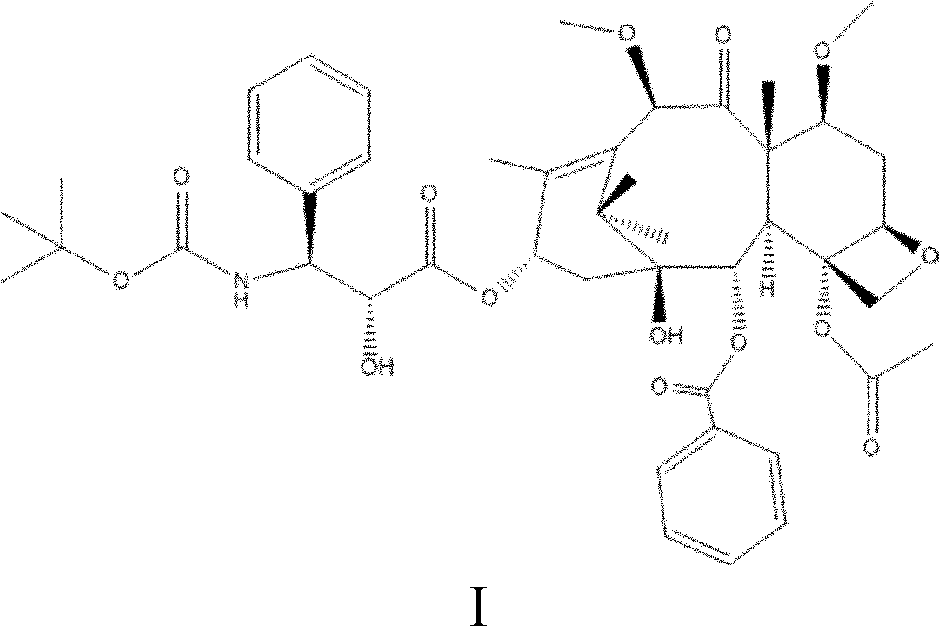
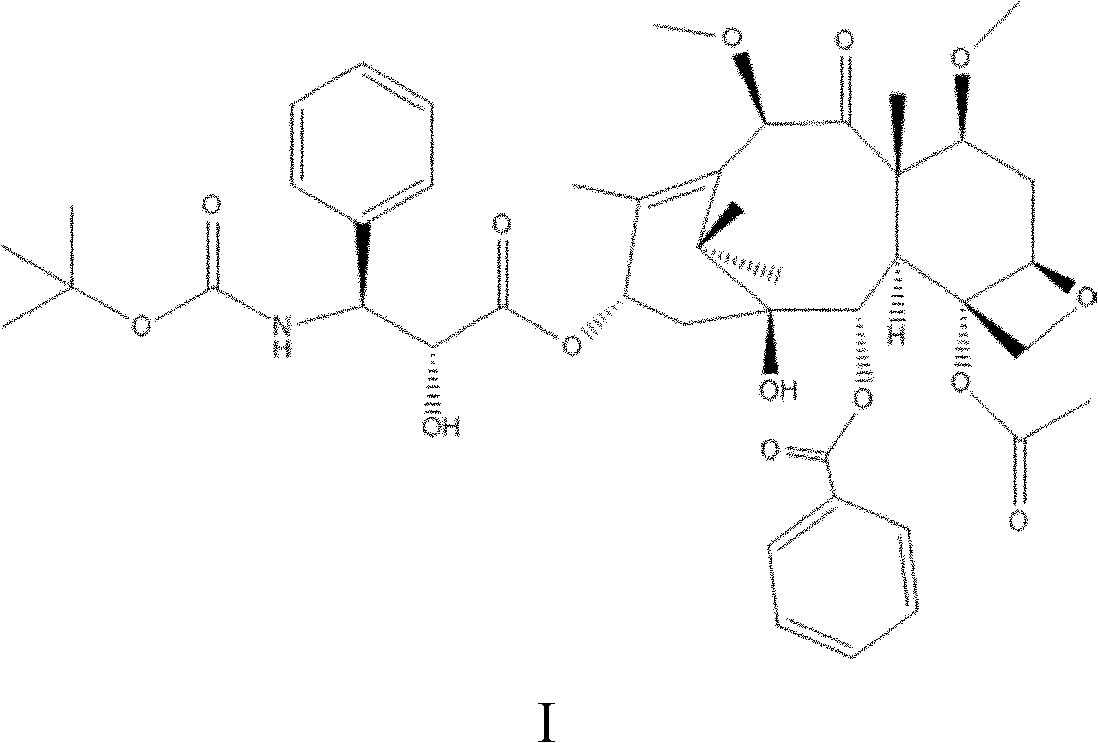
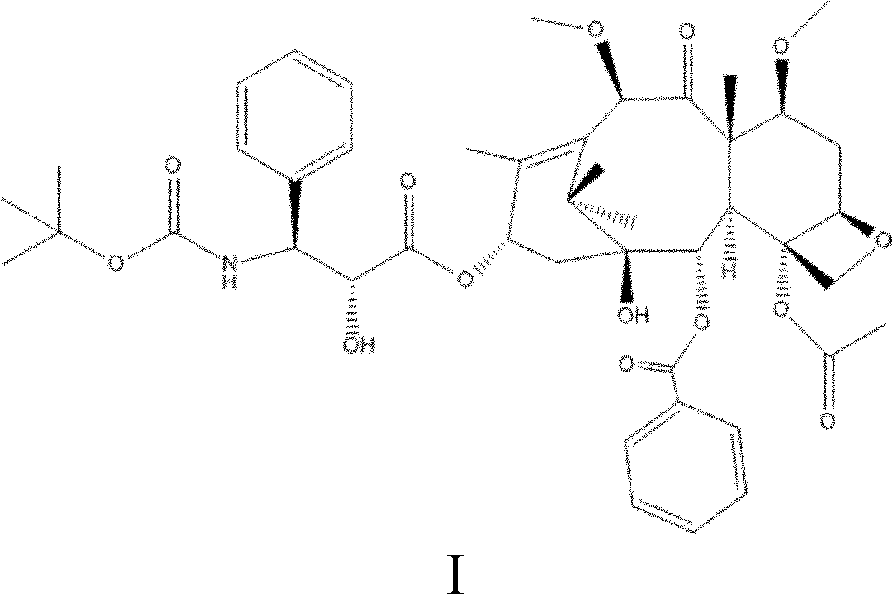
[0032] A preparation method of taxane compounds shown in formula I is under the protection of nitrogen or argon, using docetaxel as a raw material, using benzene, alcohols, ketones or ethers as a solvent, and using 0.0083mL / s Add dimethyl sulfate 1 to 10 times the molar amount of docetaxel dropwise at a rate of ~0.05mL / s, and perform alkylation reaction at 10~60°C for 0.5~10h, during which time the reaction is controlled with a weakly alkaline organic solvent The pH value of the solution is 7-8. After the reaction is completed, water is added for crystallization, and the obtained crystal is the crude compound shown in formula I. Then dissolve the crude product of the compound shown in formula I with an alcohol compound, and then add water to precipitate the crystal, and the obtained crystal is the pure product of the compound shown in formula I, wherein, every 1 g of the crude product is dissolved by adding 10-20 mL of the compound of formula I, and adding 1-10 g of water to pr...
Embodiment 1
[0035] Embodiment 1: Preparation of taxane compounds of the present invention
[0036]Under nitrogen protection, put 25.8g (0.032mol) of docetaxel into a 500mL three-necked flask, add 258ml of acetone to raise the temperature to 35°C, stir the solution until it becomes clear, then add 10g (0.08mol) of sulfuric acid dropwise at a speed of 0.0083mL / s Dimethyl ester, while adding pyridine dropwise to maintain the pH value at 7-8 (the amount of pyridine is based on the pH value), after reacting for 5 hours at 40°C, add 60g of water to precipitate crystals, filter, and dry to obtain the crude product of the compound shown in formula I 24.1 g, yield 90.63%. The crude compound shown in Formula I was stirred and dissolved in 250 mL of methanol at 30°C to 40°C, then 25g of water was added to crystallize, the crystals were collected by filtration, and dried to obtain 22.2g of the compound shown in Formula I with a yield of 92.12% and a purity of 99.3 %.
[0037] NMR test results showe...
Embodiment 2
[0038] Embodiment 2: Preparation of taxane compounds of the present invention
[0039] Under the protection of argon, put 77.5g (0.096mol) of docetaxel into a three-necked flask, add 77.5mL of ethanol and raise the temperature to 35°C, stir the solution until it becomes clear, then add 12.6g (0.10mol) of docetaxel dropwise at a speed of 0.05mL / s ) dimethyl sulfate, while adding pyridine dropwise to maintain the pH value at 7 to 8 (the amount of pyridine is based on the pH value), after reacting for 10 hours at 10°C, add 300g of water, precipitate crystals, filter, and dry to obtain the compound of formula I The crude product was 74.9g, and the yield was 93.75%. The crude compound represented by formula I was dissolved in 1500 mL of ethanol, then crystallized by adding 700 g of water, the crystals were collected by filtration, and dried to obtain 46.5 g of the compound represented by formula I, with a yield of 93% and a purity of 99.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com