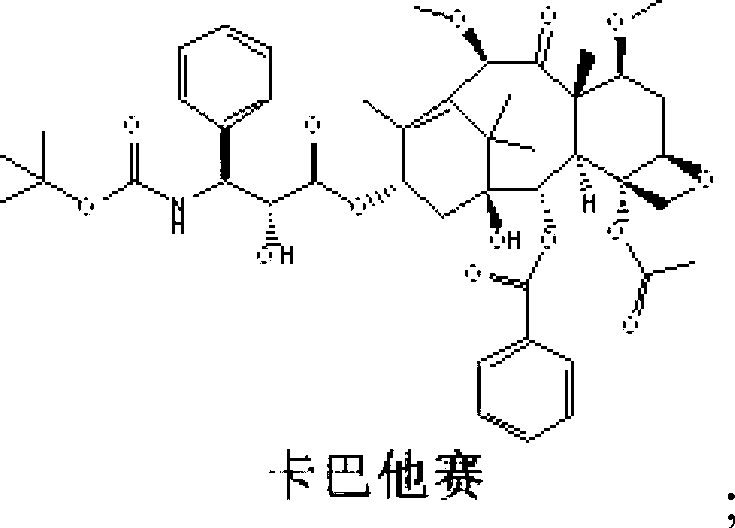
Preparation method of cabazitaxel and intermediate thereof
A technology of cabazitaxel and compounds, which is applied in the field of drug synthesis, can solve the problems of low selectivity, low yield of cabazitaxel, and unsuitability for commercial production, and achieve the effect of high yield and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
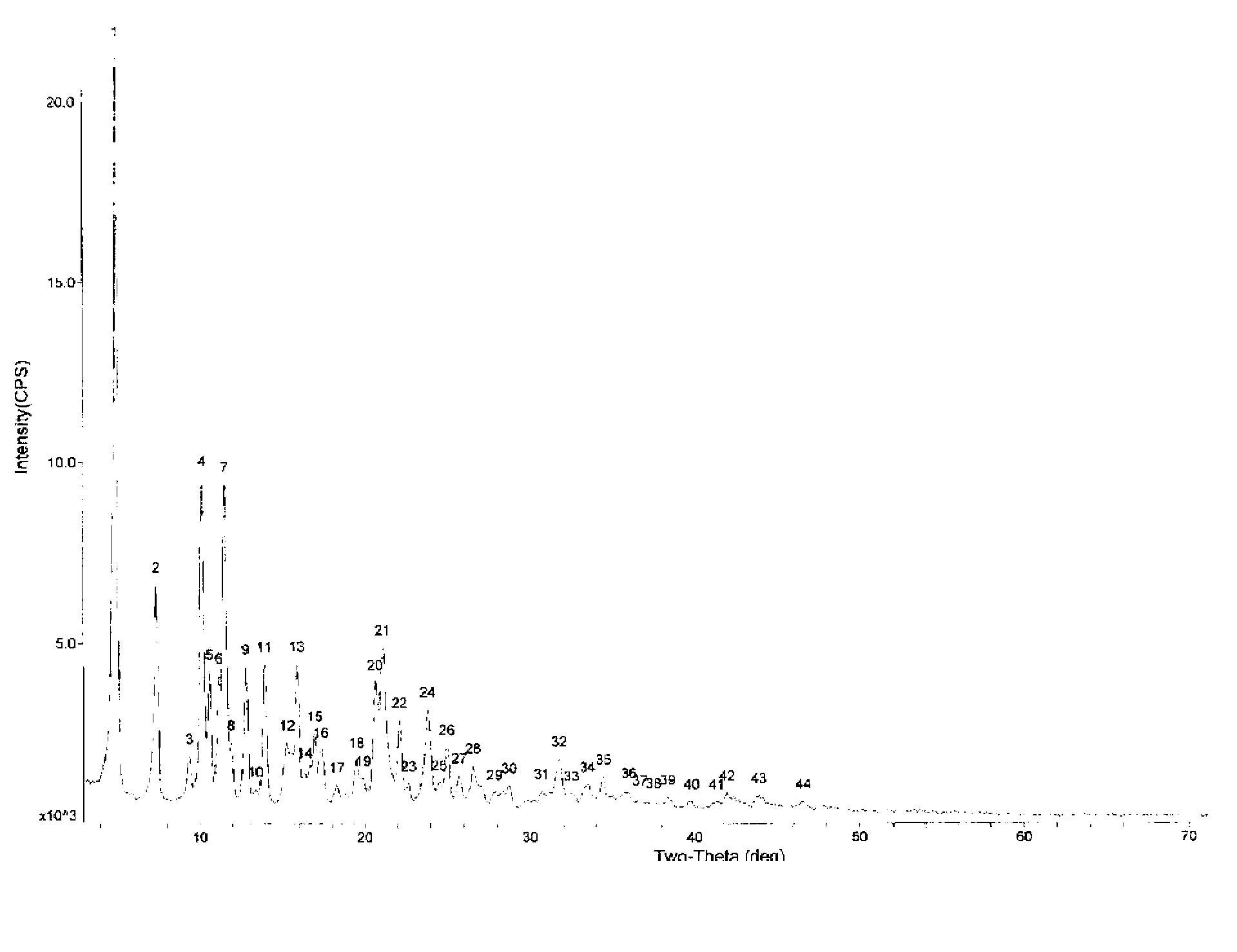
Image
Examples
Embodiment 1
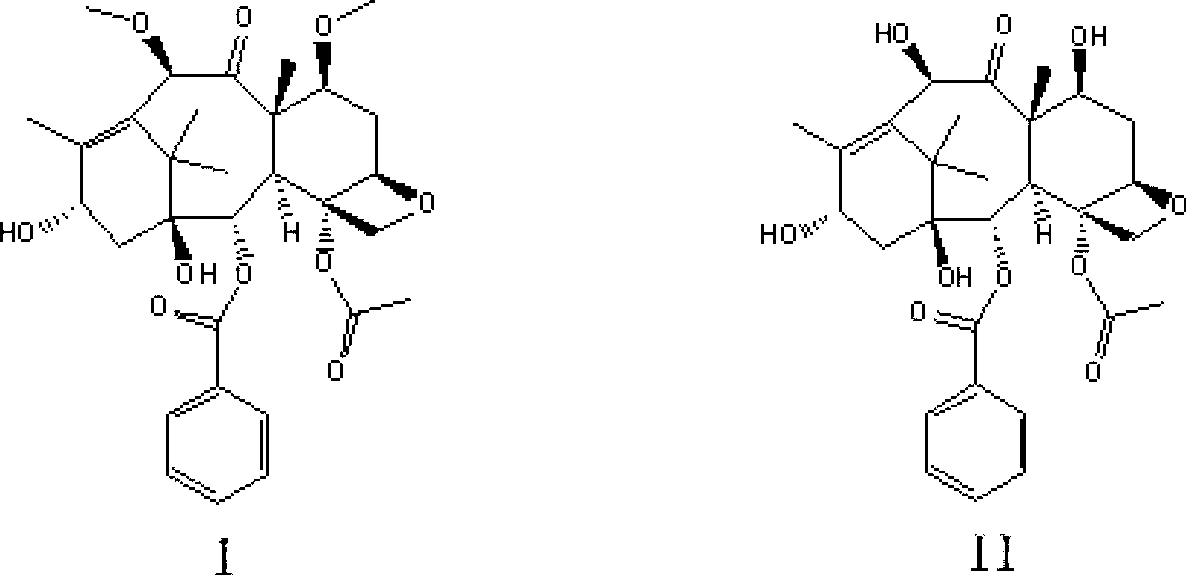
[0032] The preparation of the cabazitaxel intermediate shown in embodiment 1 formula I
[0033] Add 16.6g (30.5mmol) of the compound shown by formula II into a 2000ml three-necked flask, dissolve it in 1000ml of anhydrous tetrahydrofuran, add 10.5g (64.1mmol) of methyl triflate and cool it down to -60°C, slowly drop the concentration It is 50.8ml of 1.2M LiHMDS tetrahydrofuran solution (LiHMDS content is 61mmol). After the dropwise addition, close the freezing tank, and naturally raise the temperature to -30°C for 40min. After the reaction, add 50ml of saturated NaHCO to the reaction system 3 Aqueous solution quenched the reaction. Add a mixed solvent composed of 200ml dichloromethane and 800ml isopropyl ether to the reaction mixture, precipitate a white solid, filter, wash the solid with 30ml dichloromethane and 30ml isopropyl ether successively, and dry in vacuo to obtain a white powder which is formula I Shown compound, yield is 13.1g, and yield is 75.0%; Its spectral data...
Embodiment 2
[0035] The preparation of compound shown in embodiment 2 formula I
[0036] Add 16.6g (30.5mmol) of the compound shown by formula II into a 2000ml three-necked flask, dissolve it in 1000ml of anhydrous tetrahydrofuran, add 7.3g (64.1mmol) of methyl fluorosulfonate and cool it down to -60°C, slowly drop the concentration to 1.2 M LiHMDS tetrahydrofuran solution 50.8ml (LiHMDS content is 61mmol), after the dropwise addition, close the freezer, naturally warm to -30 ° C for 40min, after the reaction, add 50ml saturated NaHCO to the reaction system 3 Aqueous solution quenched the reaction. Add 200ml of dichloromethane and 800ml of isopropyl ether into the mixed solvent of the reaction mixture, a white solid precipitates, filters, washes the solid with 30ml of dichloromethane and 30ml of isopropyl ether, and vacuum-dries to obtain a white powder of formula Compound shown in I, output is 12.2g, and yield is 70.0%; Its spectral data are with embodiment 1.
Embodiment 3
[0037] The preparation of compound shown in embodiment 3 formula I
[0038] Add 16.6g (30.5mmol) of the compound shown by formula II into a 2000ml three-neck flask, dissolve it in 1000ml tetrahydrofuran, add 7.1g (64.1mmol) of methyl methanesulfonate and cool it down to -60°C, slowly dropwise add 1.0M NaHMDS tetrahydrofuran solution 61.0ml (NaHMDS content is 61.0mmol), after the dropwise addition, close the freezing tank, naturally warm up to -30°C for 40min, after the reaction, add 50ml saturated NaHCO 3 Aqueous solution quenched the reaction. Add a mixed solvent composed of 200ml dichloromethane and 800ml isopropyl ether to the reaction mixture, precipitate a white solid, filter, wash the solid with 30ml dichloromethane and 30ml isopropyl ether successively, and dry in vacuo to obtain a white powder which is formula I Shown compound, yield is 14.3g, and yield is 81.9%; Its spectral data are with embodiment 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com