Crystal forms of cabazitaxel and preparation method thereof
A technology of cabazitaxel and forms, applied in organic chemical methods, medical preparations containing active ingredients, pharmaceutical formulations, etc., can solve the differences in microscopic crystal structures that affect the processing performance, appearance, physical and chemical properties and biological activities of preparations and other problems, to achieve the effects of weak toxicity, good storage and handling stability, and excellent performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
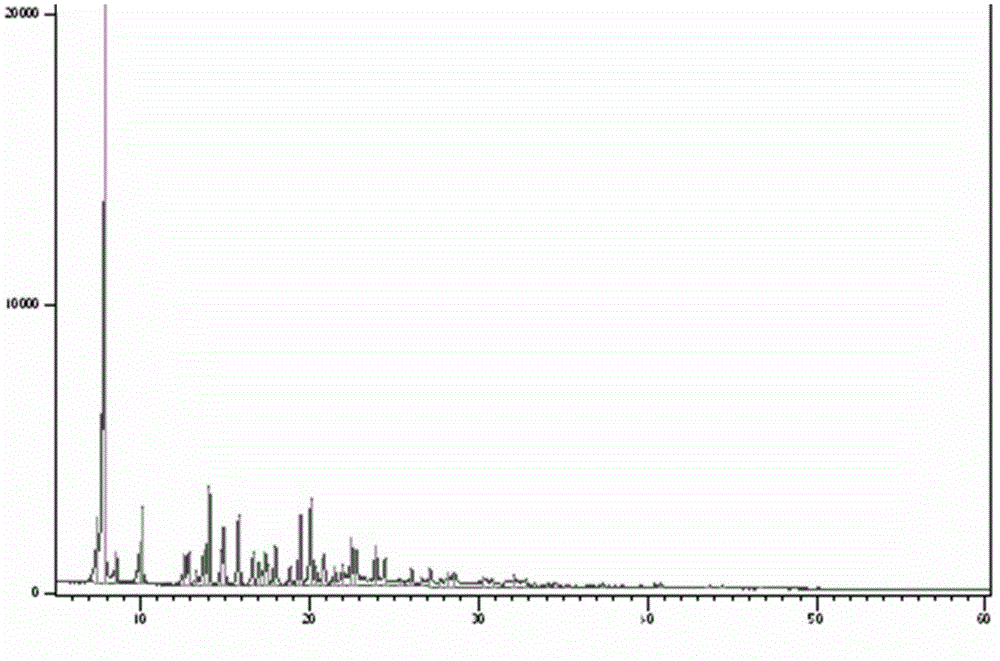
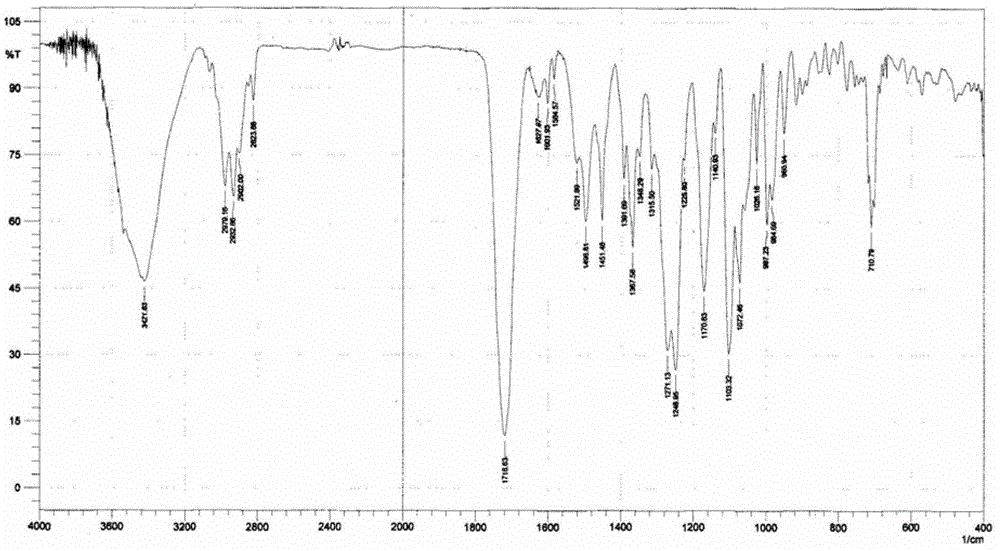
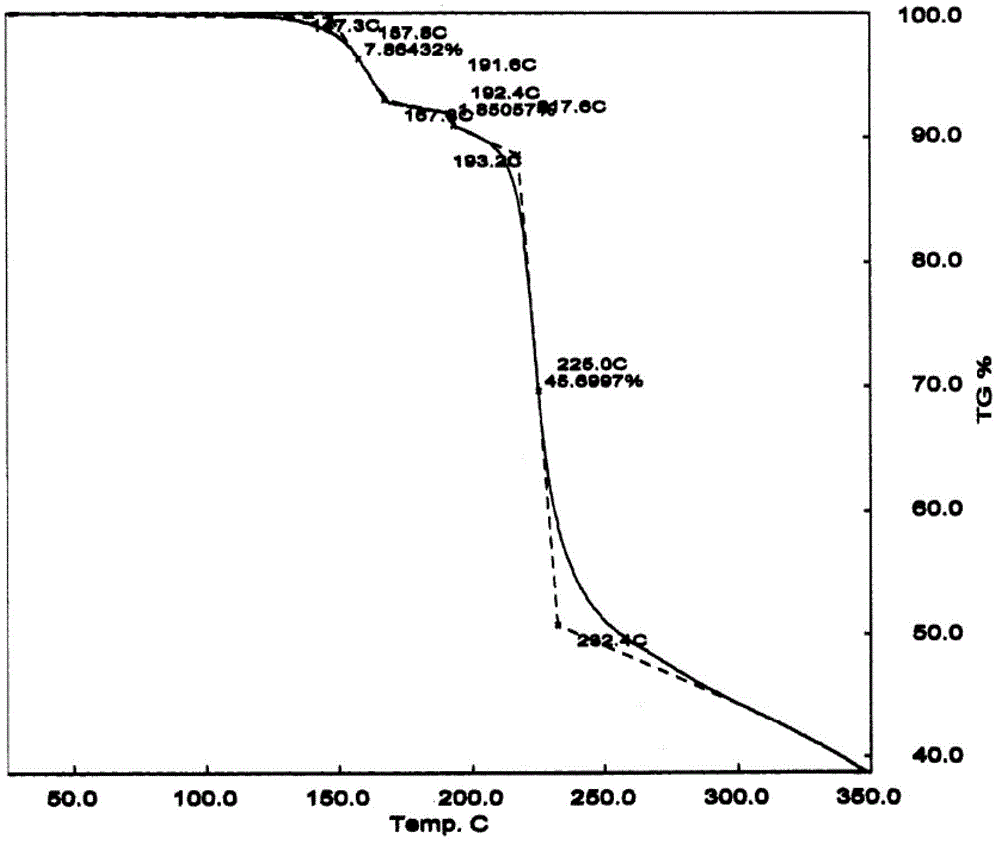
[0051] Add 10 g of cabazitaxel monohydrate to 300 mL of ethyl acetate and heat to dissolve, concentrate until a small amount of crystals precipitate out, crystallize overnight at 0-5°C, filter with suction, and vacuum-dry the filter cake at a temperature of 50-60°C to obtain 9.4 g of cabazitaxel ethyl acetate compound form type J crystal. HPLC detection purity ≥ 99.76%, GC detection of its ethyl acetate residue is 95000ppm, X-ray powder diffraction spectrum, infrared spectrum spectrum, TGA spectrum and DSC spectrum respectively see figure 1 , figure 2 , image 3 , Figure 4 .
Embodiment 2
[0053] Add 10 g of cabazitaxel to 300 mL of methyl formate and heat to dissolve, concentrate until a small amount of crystals precipitate out, crystallize overnight at 0-5°C, filter with suction, and vacuum-dry the filter cake at a temperature of 50-60°C to obtain 9.0 g of cabazitaxel The form of methyl tacetidine carboxylate is crystal J. The purity detected by HPLC is ≥99.78%, and the residual methyl formate is 85400ppm detected by GC.
Embodiment 3
[0055] Add 5g of cabazitaxel ethyl acetate into 75mL of ethanol and stir to dissolve, then quickly add 150mL of purified water dropwise, cool down to 8°C after the addition, let stand for 2h and then filter, wash the filter cake with 200mL of purified water for several times, The filter cake was dried under reduced pressure at 60°C for 12 hours to obtain 4.6 g of white crystalline cabazitaxel monohydrate. HPLC detection purity ≥ 99.87%, Karl Fischer method determination of water content of 2.4%, its X-ray powder diffraction spectrum, infrared spectrum, TGA spectrum and DSC spectrum are shown in Figure 5 , Figure 6 , Figure 7 , Figure 8 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com