Cabazitaxel injection and preparation method thereof
A technology for injections and preparations, which is applied in the direction of pharmaceutical formulas, medical preparations with non-active ingredients, and medical preparations containing active ingredients, etc. To ensure the accuracy of clinical dosage and other issues, to achieve the effects of shortening production time, simplifying clinical preparation methods, enhancing stability and dosage accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Embodiment 1: prepare Cabazitaxel injection
[0023] (1) Preparation composition:
[0024] Cabazitaxel 0.6g
[0025] Polysorbate 80 15.6g
[0026] Citric acid 0.02g
[0027] Absolute ethanol 11.9g
[0028]
[0029] Total 30ml
[0030] (2) Preparation method:
[0031] Dissolve 0.6 g of cabazitaxel and 0.02 g of citric acid in 10.7 g of absolute ethanol, add 15.6 g of polysorbate 80, stir evenly, add 1.2 g of absolute ethanol to the full amount of 30 ml, continue stirring, and take the intermediate to test the pH, pH Be 4.91; Carry out sterilizing filtration by 0.22 μm microporous membrane, filtrate is aseptically filled in 10ml vial with every 3ml, corkscrew, makes the preparation of cabazitaxel injection (specification 60mg / ).
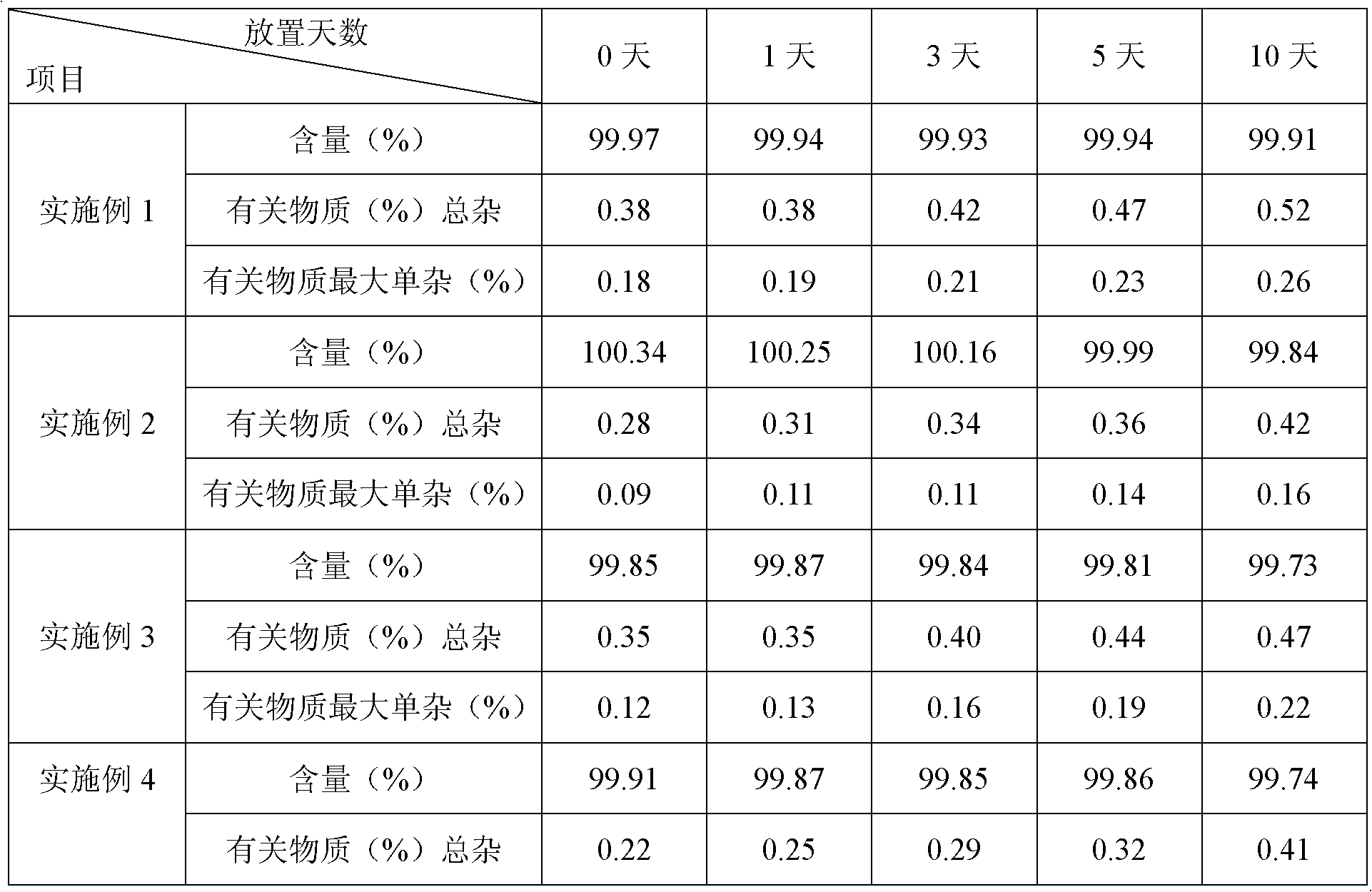
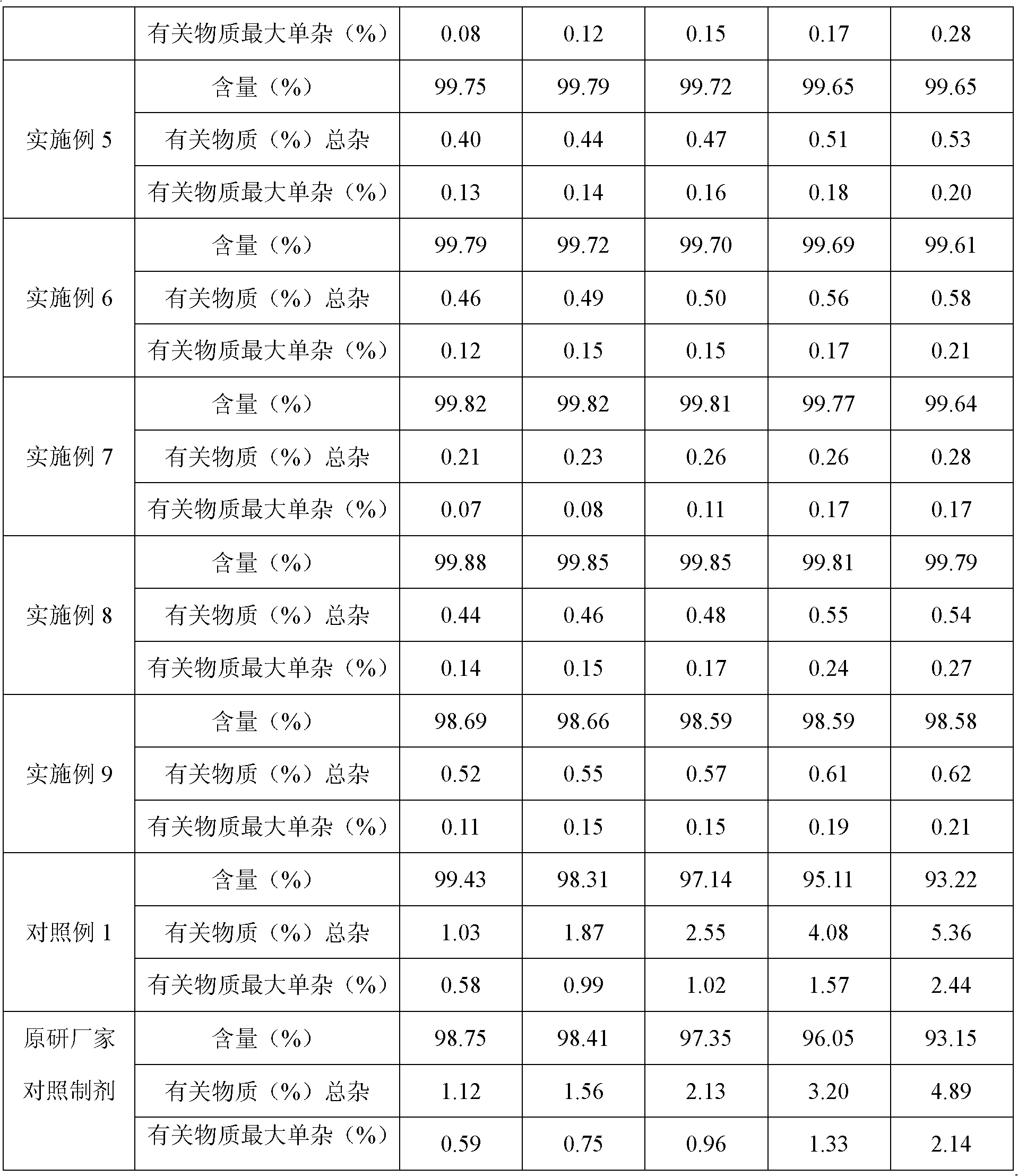
[0032] The purity of the product detected by HPLC is 99.62% (0.38% of the total impurities of related substances), and the maximum single impurity is 0.18%;
[0033] HPLC detection conditions are:...
Embodiment 2
[0042] Embodiment 2: prepare Cabazitaxel injection
[0043] (1) Preparation composition:
[0044] Cabazitaxel 0.2g
[0045] Polysorbate 80 16g
[0046] Citric acid 1mg
[0047] Absolute ethanol 4g
[0048]
[0049]Total 20ml
[0050] (2) Preparation method:
[0051] Dissolve 0.2 g of cabazitaxel and 1 mg of citric acid in 3.2 g of absolute ethanol, add 16 g of polysorbate 80, stir evenly, add 0.8 g of absolute ethanol to the full amount of 20 ml, continue stirring, take the intermediate to test the pH, pH Be 6.0; Carry out sterilizing filtration by 0.22 μm microporous membrane, filtrate is aseptically filled in 10ml vial with every 2ml, corkscrew, forms the preparation of cabazitaxel injection (specification 20mg / ).
[0052] The purity of the product detected by HPLC is 99.72% (the total impurities of related substances are 0.28%), and the maximum simple impurity is 0.09%; the HPLC detection conditions are the same as in Example 1.
Embodiment 3
[0053] Embodiment 3: prepare Cabazitaxel injection
[0054] (1) Preparation composition:
[0055] Cabazitaxel 1.2g
[0056] Polysorbate 80 6g
[0057] Citric acid 0.1g
[0058] Absolute ethanol 11.3g
[0059]
[0060] Total 20ml
[0061] (2) Preparation method:
[0062] Dissolve 1.2 g of cabazitaxel and 0.1 g of citric acid in 9 g of absolute ethanol, add 6 g of polysorbate 80, stir evenly, add 2.3 g of absolute ethanol to the full amount of 20 ml, continue stirring, and take the intermediate to test the pH, pH Be 4.43; Carry out sterilizing filtration by 0.22 μm sterilizing filter ball, filtrate is aseptically filled in the 10ml vial with every 2ml, corkscrew, forms the preparation of cabazitaxel injection (specification 120mg / ).
[0063] The purity of the product detected by HPLC is 99.65% (0.35% of the total impurities of related substances), and the maximum simple impurity is 0.12%; the HPLC detection conditions ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com