Multiphase-stable albumin conjunction type cabazitaxel
A technology of albumin-binding type and cabazitaxel, which is applied in the direction of drug combination, medical preparations containing active ingredients, non-active ingredients of polymer compounds, etc. It can solve problems such as many influencing factors, difficulty in scale-up production, stability research, etc. , to achieve the effects of fewer process influencing factors, strict temperature control, and improved stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Ratio of components in heterogeneously stable albumin-bound cabazitaxel (mg)
[0031]
[0032]
[0033] Preparation:
[0034] (1) Dissolving cabazitaxel, phospholipid and citric acid in an appropriate amount of chloroform / dichloromethane as the oil phase;
[0035] (2) Dissolving the prescription amount of albumin, phospholipids and mannitol in an appropriate amount of deionized water as the water phase;
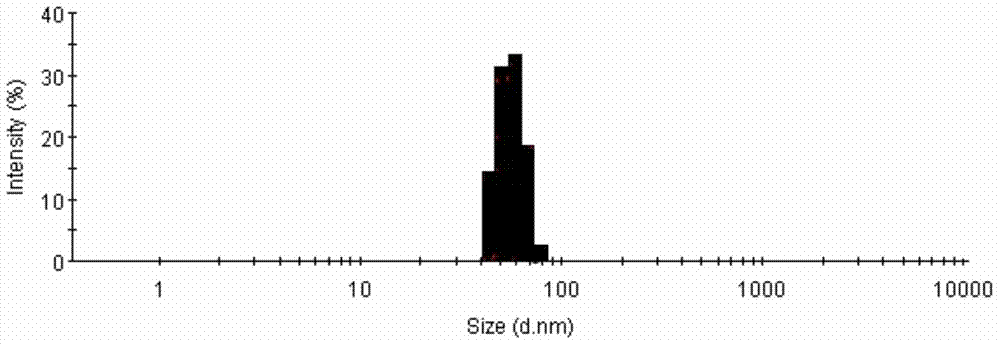
[0036] (3) Add the oil phase prepared in (1) dropwise to (2), and grind it using a temperature-controllable planetary ball mill (ball milling speed 38Hz, ball milling temperature 4°C, ball milling time 1.5 hours), take out, pour After lyophilization, a heterogeneous and stable albumin-bound cabazitaxel was obtained. The particle size was measured using a laser particle size analyzer, and the result was 321 nm.
Embodiment 2
[0038] Ratio of components in heterogeneously stable albumin-bound cabazitaxel (mg)
[0039]
[0040] Preparation:
[0041] (1) Dissolving cabazitaxel, PEG-PLGA and citric acid in an appropriate amount of chloroform / dichloromethane as the oil phase;
[0042] (2) dissolving the prescribed amount of albumin, PEG-PLGA, and mannitol in an appropriate amount of deionized water as the water phase;
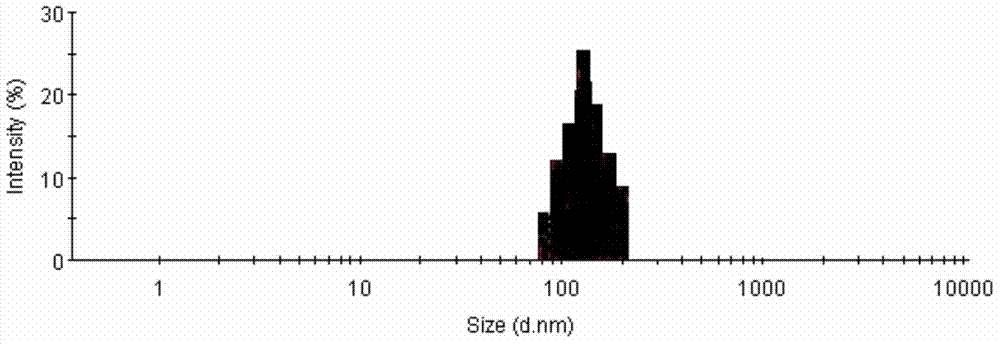
[0043] (3) Add the oil phase prepared in (1) dropwise to (2), grind it with a temperature-controllable planetary ball mill, take it out, fill it, and freeze-dry it to obtain a multiphase stable albumin-binding type Cabernet. The particle size was measured using a laser particle size analyzer, and the result was 386 nm.
Embodiment 3
[0045] Ratio of components in heterogeneously stable albumin-bound cabazitaxel (mg)
[0046]
[0047] Preparation:
[0048] (1) Dissolving cabazitaxel, PEG-PLGA and citric acid in an appropriate amount of chloroform / dichloromethane as the oil phase;
[0049] (2) dissolving the prescribed amount of albumin, PEG-PLGA, and mannitol in an appropriate amount of deionized water as the water phase;
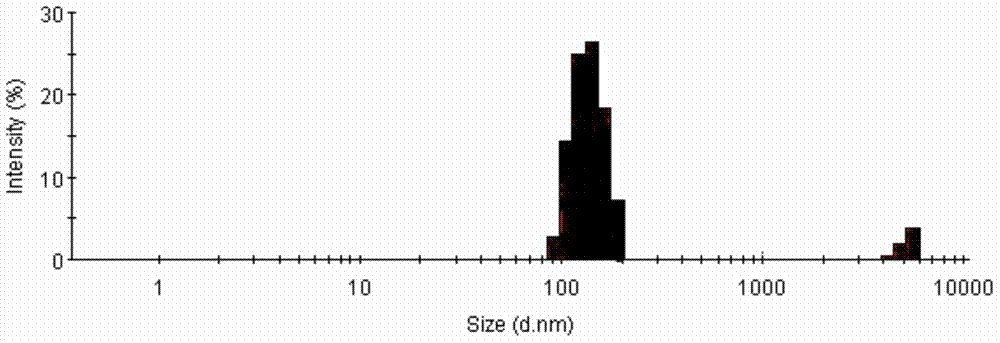
[0050] (3) Add the oil phase prepared in (1) dropwise to (2), grind it with a temperature-controllable planetary ball mill, take it out, fill it, and freeze-dry it to obtain a multiphase stable albumin-binding type Cabernet. The particle size was measured using a laser particle size analyzer, and the result was 263 nm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com