Method for preparing cabazitaxel
A technology of cabazitaxel and compounds, which is applied in the field of preparation of cabazitaxel, can solve the problems of unfavorable large-scale production, increased production cost, and long reaction time, and achieve the effect of facilitating industrial production, reducing reaction steps, and shortening the reaction cycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
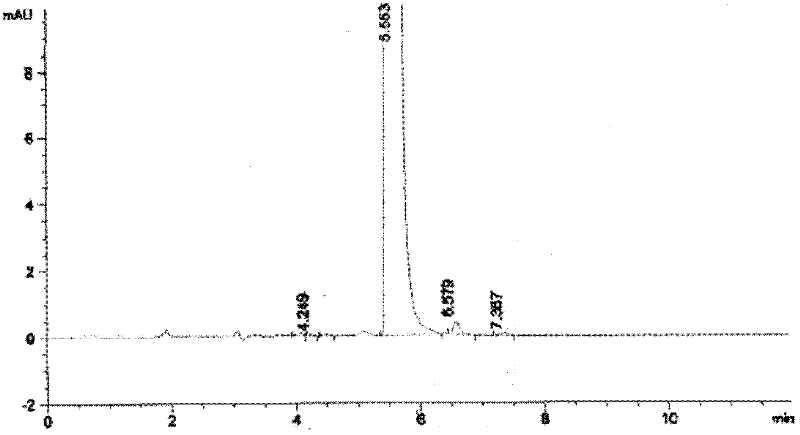
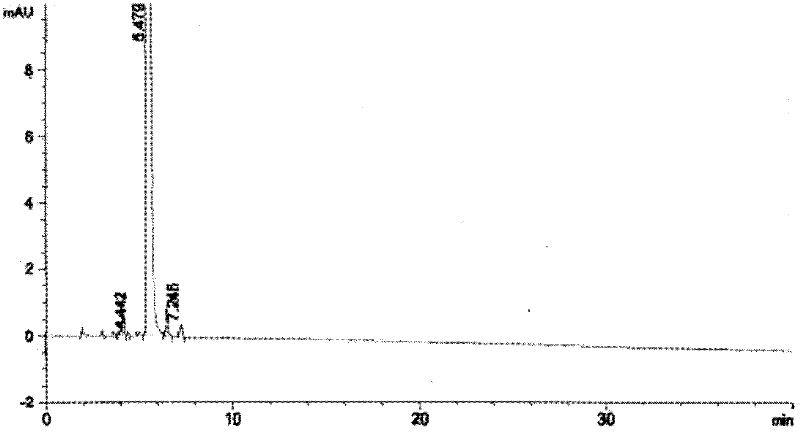
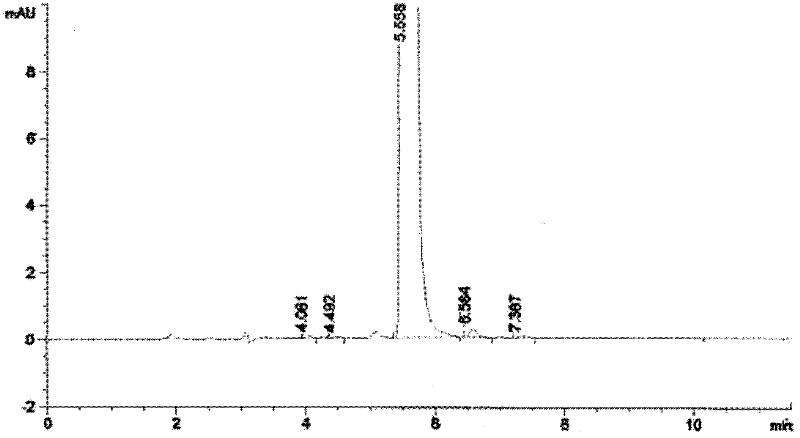
Image
Examples
preparation example Construction
[0040] The invention discloses a preparation method of cabazitaxel, and those skilled in the art can learn from the content of this article and appropriately improve the process parameters to realize it. In particular, it should be pointed out that all similar replacements and modifications are obvious to those skilled in the art, and they are all considered to be included in the present invention. The method and application of the present invention have been described through preferred embodiments, and the relevant personnel can obviously make changes or appropriate changes and combinations to the method and application described herein without departing from the content, spirit and scope of the present invention to realize and Apply the technology of the present invention.
[0041] The invention provides a preparation method of cabazitaxel, comprising the steps of:
[0042] Step 1: In an ether solvent or DMSO, DMF, DMA, after mixing the compound shown in formula II with exc...
Embodiment 1
[0073] Dissolve 10g of the compound represented by formula II in 200mL of anhydrous tetrahydrofuran, put it in a freezing tank, cool down to -30°C, start to add 30mL of tetrahydrofuran solution of sodium bistrimethylsilamide dropwise, after the addition, the temperature is -28 ℃, add 8mL dimethyl sulfate dropwise, after the dropwise addition, close the freezing tank, naturally raise the temperature to -15°C and react for 10 hours, after the reaction, add 100mL saturated sodium bicarbonate, then add 60mL 20% glacial acetic acid aqueous solution, measure the pH =5, the reaction solution was distilled to dryness, after adding 300mL dichloromethane and 200mL saturated sodium chloride for dissolving, the layers were extracted, the organic layer was collected, and the distillation ended to obtain 9g crude product, and the crude product was used for silica gel chromatography column (eluent was After chromatography with ethyl acetate and n-hexane), 6.7 g of the compound represented by ...
Embodiment 2
[0077] Dissolve 10g of the compound represented by formula II in 220mL of anhydrous tetrahydrofuran, put it in a freezing tank, cool down to -30°C, start to add 30mL of tetrahydrofuran solution of sodium bistrimethylsilamide dropwise, after the addition, the temperature is -28 ℃, add 7.8mL dimethyl sulfate dropwise, after the dropwise addition, close the freezing tank, naturally raise the temperature to -15°C for 15 hours, after the reaction, add 100mL saturated sodium bicarbonate, and then add 65mL of 20% glacial acetic acid aqueous solution Measure PH=5, the reaction solution is distilled to dryness, after adding dichloromethane and saturated sodium chloride to dissolve, extract and separate layers, collect the organic layer, the distillation finishes and obtains 8.8g crude product, the crude product is purified by silica gel chromatography column (eluent (Ethyl acetate and n-hexane) chromatography to obtain 6.5 g of the compound represented by formula III as off-white solid,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com