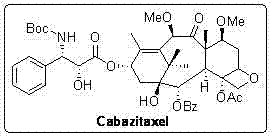
The synthetic method of cabazitaxel
A technology of cabazitaxel and isoborneol, which is applied in the field of drug synthesis, can solve problems such as no description of the synthesis method of cabazitaxel, and achieve the effect of short steps and mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0024] The preparation method of cabazitaxel of the present invention, its synthetic theoretical analysis is as follows:
[0025]
[0026] Reaction scheme 2. Retrosynthesis analysis of cabazitaxel
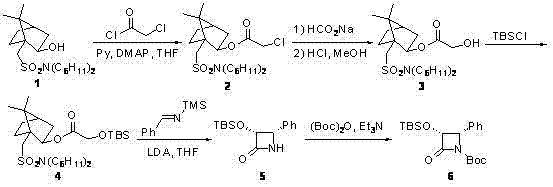
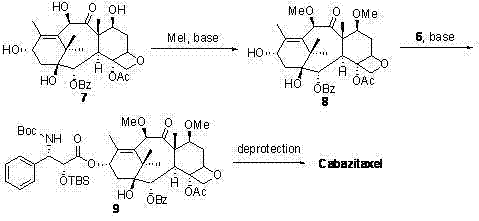
[0027]From the retrosynthetic analysis (reaction formula 2), it can be seen that chiral β-lactam and 7,10-dimethoxy-10-baccatin III (7,10-dimethoxy-10-deacetylbaccatin III) are the most suitable for the synthesis of carbastatin Two key intermediates of race. Referring to literature method (Tetrahedron, 1992, 48, 6985-7012; 1996, 52, 209-224), we use Oppolzer reagent 1 As a chiral source, after five steps of reaction, (3R,4S)-β-lactam with 98% ee was obtained 5 (reaction formula 1). Oppolzer reagent 1 Can be recycled and reused.
Embodiment 1
[0030] one, (3R,4S)-1-(tert-butoxycarbonate group)-3-(tert-butyldimethylsilyloxy)-4-phenylazetidin-2-one ( 6 ) preparation:
[0031] Step 1: Isoborneol 1 (7.5g, 18.9 mmol), anhydrous pyridine (1.53 mL, 18.9 mmol) and DMAP (50 mg) were dissolved in anhydrous tetrahydrofuran (80 mL), cooled to 0°C in an ice-water bath, and chlorinated Acetyl chloride (1.50 mL, 18.9 mmol). The ice-water bath was removed and the reaction mixture was stirred at reflux for 16 hours. When the reaction mixture was cooled to room temperature, it was slowly poured into ice-cold saturated sodium bicarbonate solution (150 mL), and extracted with diethyl ether (4×100 mL). The combined ether extracts were washed with saturated copper sulfate solution (3×50 mL) and saturated brine (2×50 mL), respectively, and dried over anhydrous sodium sulfate. The crude product was separated by flash silica gel column chromatography (eluted with 10% ethyl acetate / n-hexane) to give isobornyl chloroacetate as a whi...
Embodiment 2
[0043] one, (3R,4S)-1-(tert-butoxycarbonate group)-3-(tert-butyldimethylsilyloxy)-4-phenylazetidin-2-one ( 6 ) preparation:
[0044] Step 1: Isoborneol 1 (7.5g, 18.9 mmol), anhydrous pyridine (1.53 mL, 18.9 mmol) and DMAP (50 mg) were dissolved in anhydrous tetrahydrofuran (80 mL), cooled to 0°C in an ice-water bath, and chlorinated Acetyl chloride (3.0 mL, 38 mmol). The ice-water bath was removed, and the reaction mixture was stirred at reflux for 15 hours. When the reaction mixture was cooled to room temperature, it was slowly poured into ice-cold saturated sodium bicarbonate solution (150 mL), and extracted with diethyl ether (4×100 mL). The combined ether extracts were washed with saturated copper sulfate solution (3×50 mL) and saturated brine (2×50 mL), respectively, and dried over anhydrous sodium sulfate. The crude product was separated by flash silica gel column chromatography (eluted with 10% ethyl acetate / n-hexane) to give isobornyl chloroacetate as a wh...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com