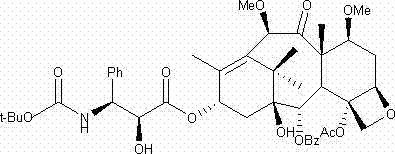
Method for purifying cabazitaxel
A technology of cabazitaxel and purity, which is applied in organic chemistry and other fields, can solve problems such as the complex chemical structure of cabazitaxel, and achieve the effects of reducing production costs, improving production efficiency, and being convenient to use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
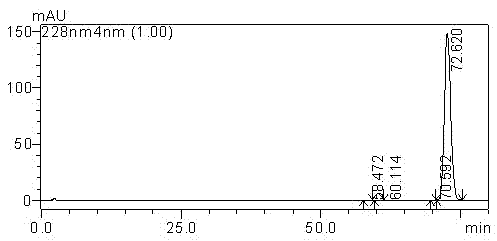
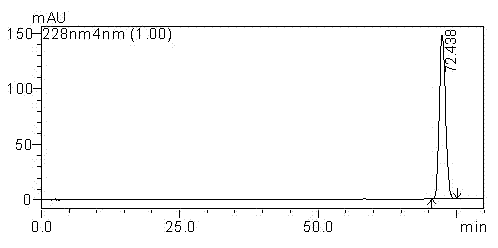
[0050]Dissolve 0.2 g of cabazitaxel with a purity of 99.5% in a mixed solvent of 100 ml of ethyl acetate and ethanol; stir and dissolve at a stirring speed of 150 rpm; the ratio of ethyl acetate and ethanol is 10:1; Add cyclohexane to the taxel solution and stir until turbidity occurs. The temperature in this process is maintained at 50°C. After turbidity is generated, the temperature is slowly lowered to 20°C. The cooling time is 3 hours, and the holding time is 3 hours for crystallization; filter, The crystallized sample was washed with purified water, and the washed sample was dried at 50° C. under reduced pressure for 2 hours. The dried sample was dissolved in 200 ml of methanol, 2.0 g of silica gel was added to the methanol solution of cabazitaxel, the stirring speed was 150 rpm, the adsorption time was 1 hour, filtered, and the sample after evaporating to dryness was placed under reduced pressure at 50°C Drying for 3 hours under high pressure liquid chromatography can ob...
Embodiment 2
[0052] Dissolve 0.25 g of cabazitaxel with a purity of 99.5% in a mixed solvent of 100 ml of dichloromethane and acetone; stir and dissolve at a stirring speed of 100 rpm; the ratio of dichloromethane and acetone is 15:1, and then add cabazitaxel Petroleum ether was added to the solution and stirred until turbidity occurred. The temperature during this process was maintained at 55°C. After turbidity occurred, the temperature was slowly lowered to 25°C within 3 hours, and the temperature was kept for 3 hours to carry out crystallization. The crystallized sample was filtered and washed, and the washed sample was dried at 40° C. under reduced pressure for 2 hours. Dissolve the dried sample in 200 ml of ethanol, add 1.0 g of silica gel to the ethanol solution of cabazitaxel, stir at 100 rpm, and absorb for 2 hours, filter, and evaporate the sample to dryness at 50 °C under reduced pressure After drying for 3 hours, 0.142 g of cabazitaxel with a single impurity content of less than...
Embodiment 3
[0054] Dissolve 0.3 g of cabazitaxel with a purity of 99.5% in 90 ml of a mixed solvent of chloroform and n-butanol; stir to dissolve at a stirring speed of 300 rpm; the ratio of chloroform and n-butanol is 8:1, and then add Add n-heptane to the taxel solution and stir until turbidity occurs. The temperature during this process is maintained at 45°C and stirred. After turbidity occurs, slowly cool down to 15°C within 3 hours and keep warm for 4 hours for crystallization. The crystallized sample was filtered and washed, and the washed sample was dried at 45° C. under reduced pressure for 2 hours. The dried sample was dissolved in 200 ml of acetone, 4.0 g of diatomaceous earth was added to the methanol solution of cabazitaxel, the stirring speed was 300 rpm, the adsorption time was 2 hours, filtered, and the sample after evaporating to dryness was desorbed at 55°C. Drying under high pressure conditions for 3 hours, the cabazitaxel 0.124g with a single impurity content of less th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com