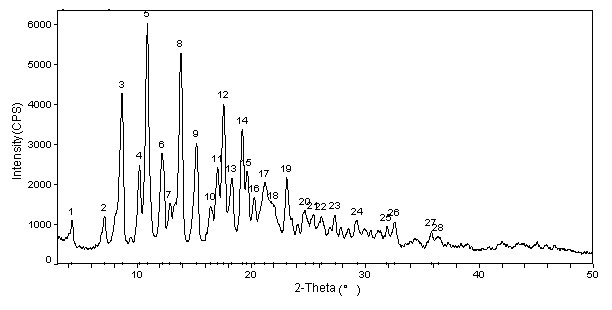
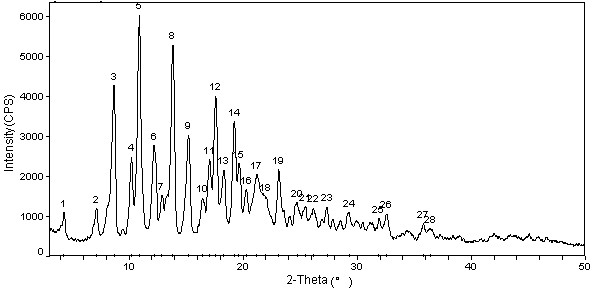
Cabazitaxel crystal and preparation method thereof
A technology of crystal and docetaxel, applied in the field of cabazitaxel crystal and its preparation, can solve the problems of harsh reaction conditions, complicated process, influence of the purity of cabazitaxel, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] 1. Dissolve 5 g of Cabazitaxel powder in 30 ml of acetone (frozen to 0°C), then slowly add the resulting solution dropwise (about 20 min) into 390 ml of distilled water (frozen to 0-3°C) while stirring, Continue to stir for 30 min after the dropwise addition, and filter with suction to obtain a white powder.
[0032] 2. The obtained white powder was dried under reduced pressure (vacuum 10-20 Pa) at room temperature for 48 h to obtain 4.4 g of white powder.
[0033] 3. Dissolve 4.5 g of the white powder obtained in operation 2 in 30 ml of acetone (frozen to 0°C), and slowly add the resulting solution dropwise (about 20 min) to 390 ml of distilled water (frozen to 0-3°C) while stirring After the dropwise addition, continue to stir for 30 min, and filter with suction to obtain a white powder.
[0034] 4. The obtained white powder was dried under reduced pressure (vacuum 10-20 Pa) at room temperature for 48 h to obtain 4.1 g of white powder with a yield of 82% wt and a pur...
Embodiment 2
[0036] 1. Dissolve 10 g of Cabazitaxel powder in 60 ml of acetone (frozen to 0°C), then slowly add the resulting solution dropwise (about 30 min) into 780 ml of distilled water (frozen to 0-3°C) while stirring, Continue to stir for 30 min after the dropwise addition, and filter with suction to obtain a white powder.
[0037] 2. The obtained white powder was dried under reduced pressure (vacuum 10-20 Pa) at room temperature for 48 h to obtain 9 g of white powder.
[0038] 3. Dissolve 9 g of the white powder obtained in operation 2 in 60 ml of acetone (frozen to 0°C), and slowly add the solution dropwise (about 30 min) to 780 ml of distilled water (frozen to 0-3°C) while stirring. After the dropwise addition, continue to stir for 30 min, and filter with suction to obtain a white powder.
[0039] 4. The obtained powder was dried under reduced pressure (vacuum 10-20 Pa) at room temperature for 48 h to obtain 8.5 g of white powder with a yield of 85% wt and a purity of 99.80 % wt....
Embodiment 3
[0042] 1. Dissolve 100 g of Cabazitaxel powder in 600 ml of acetone (frozen to 0°C), and slowly add the resulting solution dropwise (about 90 min) to 7800 ml of distilled water (frozen to 0-3°C) while stirring, After the dropwise addition, continue to stir for 50 min, and filter with suction to obtain a white powder.
[0043] 2. The obtained white powder was dried under reduced pressure (vacuum 10-20 Pa) at room temperature for 48 h to obtain 91 g of white powder.
[0044] 3. Dissolve 91 g of the white powder obtained in operation 2 in 600 ml of acetone (frozen to 0°C), and slowly add the resulting solution dropwise (about 90 min) to 7800 ml of distilled water (frozen to 0-3°C) while stirring After the dropwise addition, continue to stir for 50 min, and filter with suction to obtain a white powder.
[0045] 4. The obtained white powder was dried under reduced pressure (vacuum 10-20 Pa) at room temperature for 48 h to obtain 88 g of white powder with a yield of 88% wt and a puri...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com