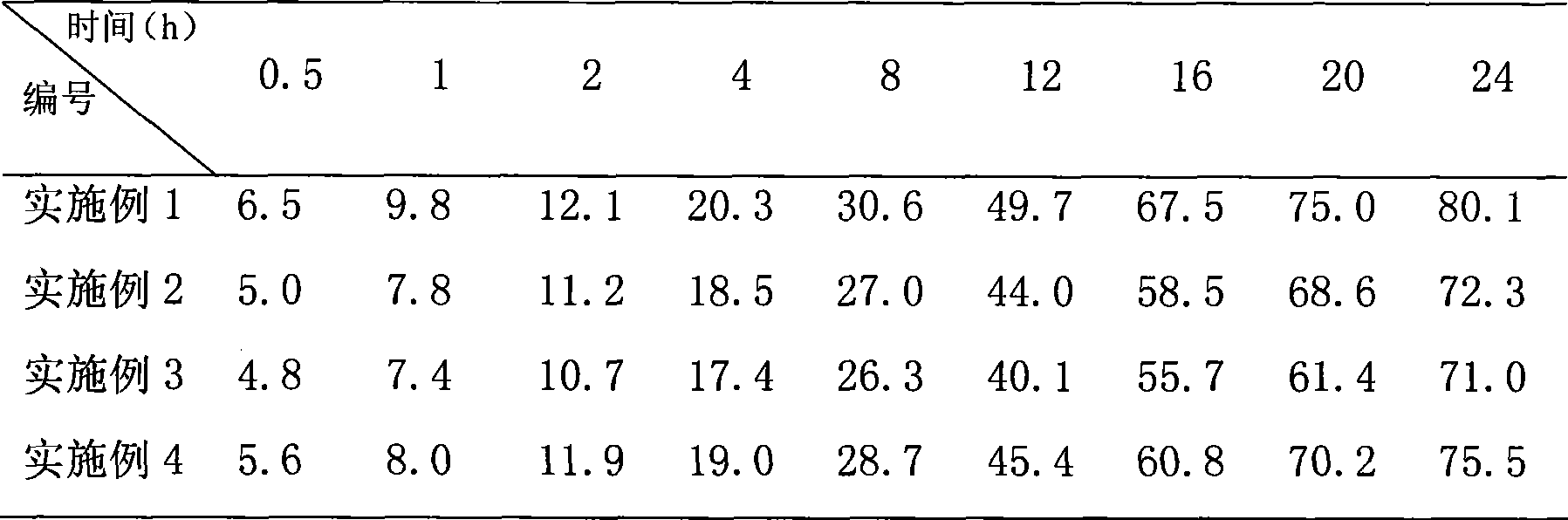
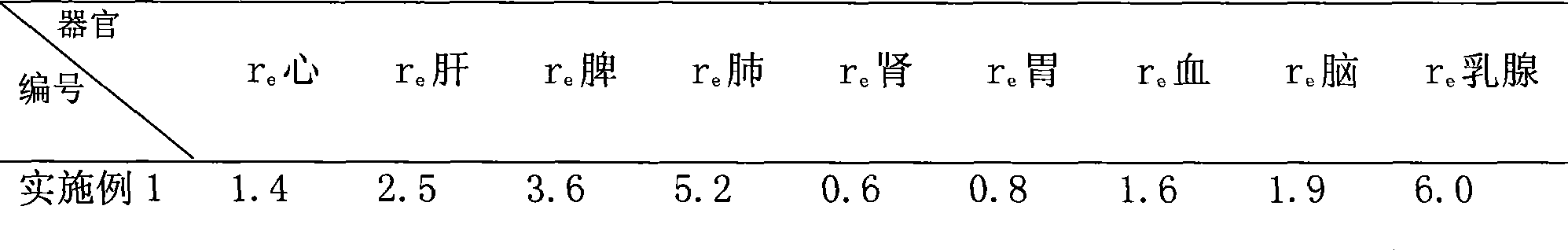
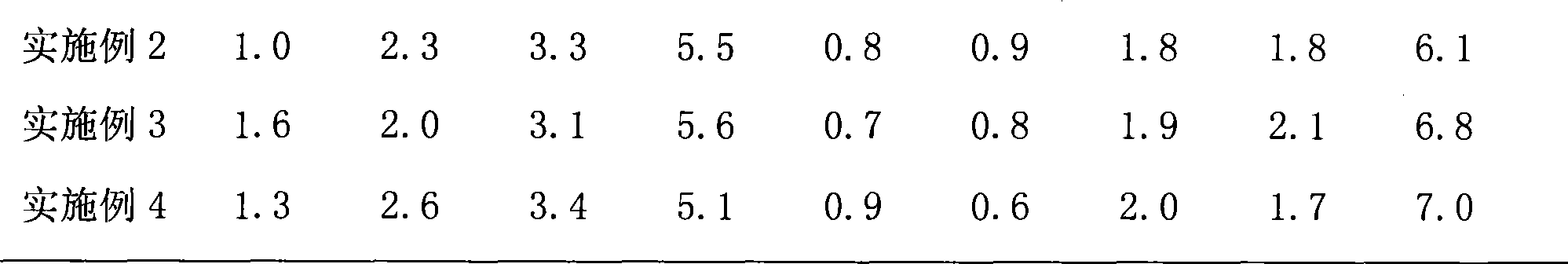
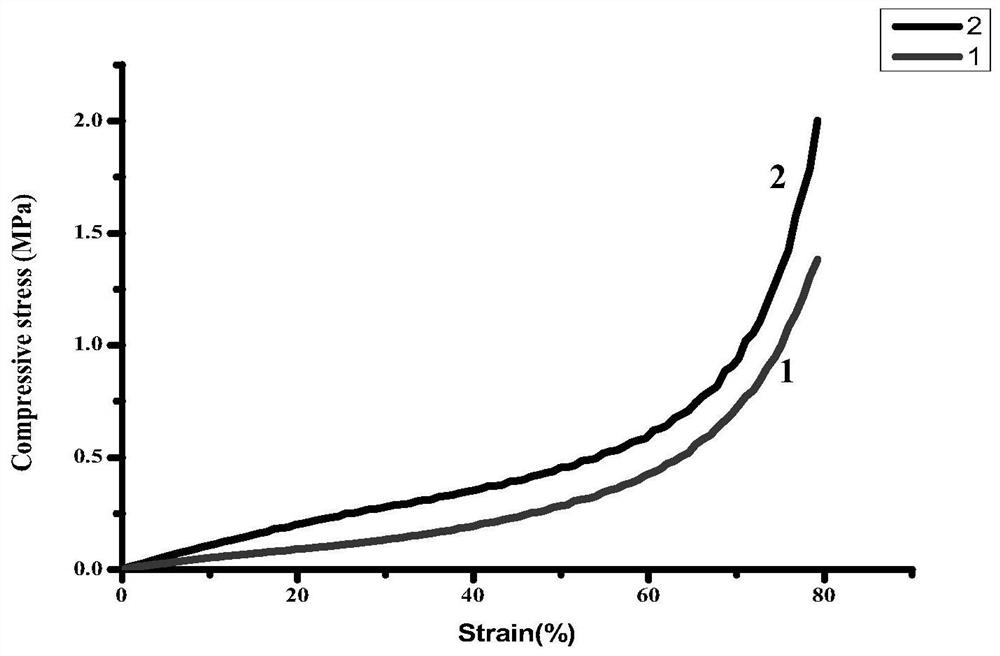
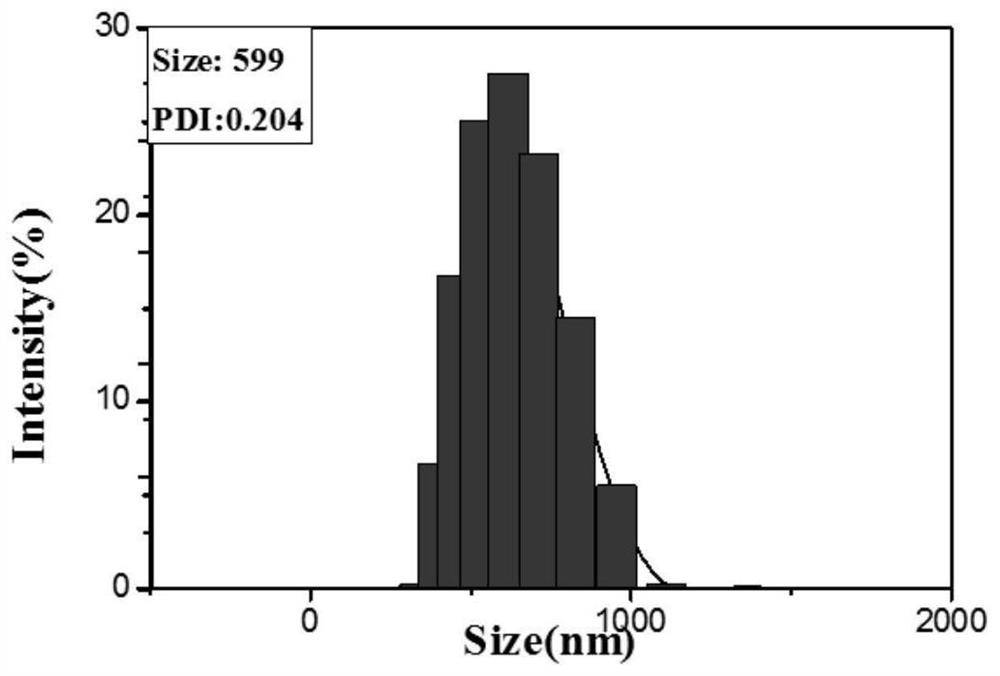
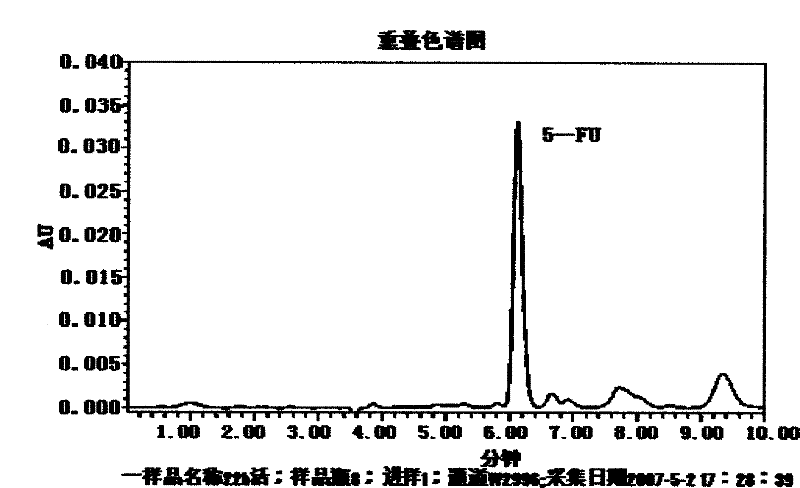
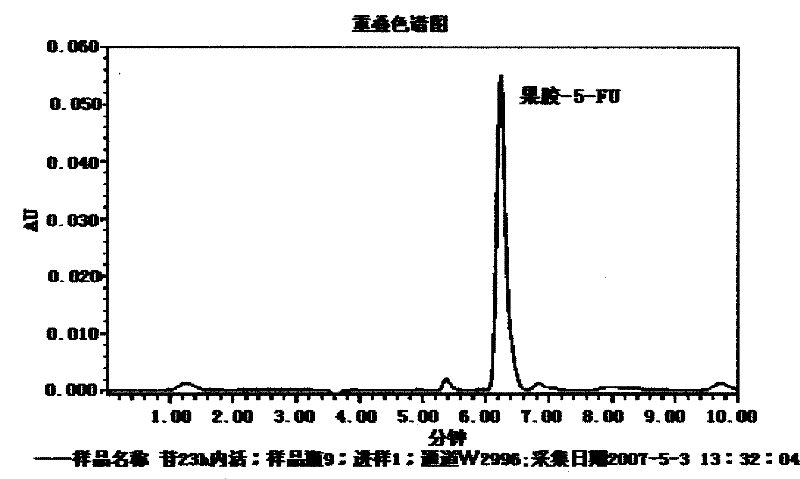
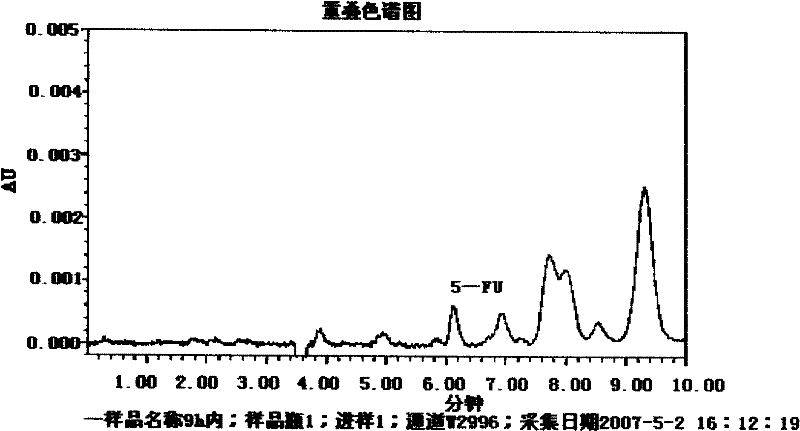
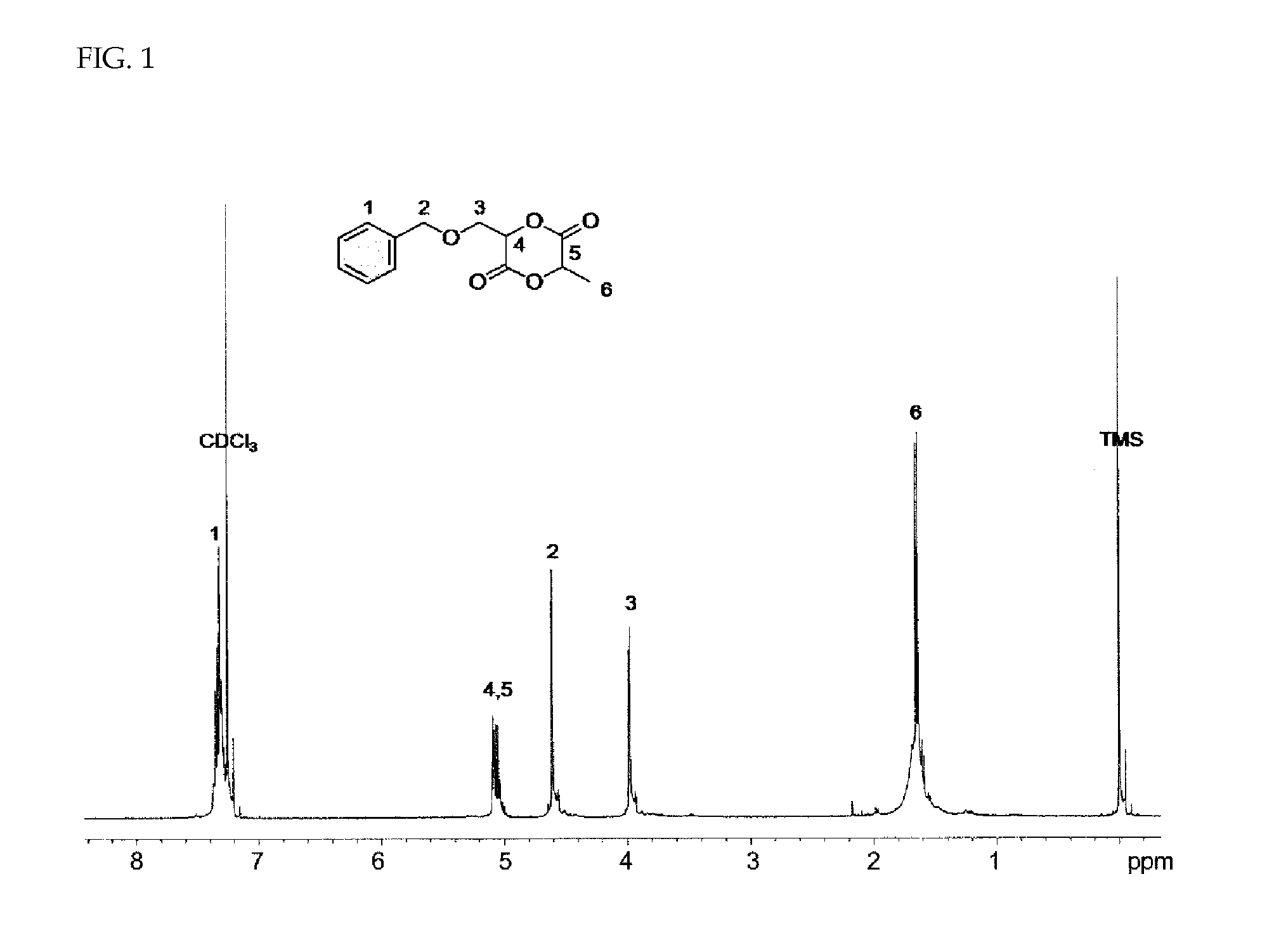
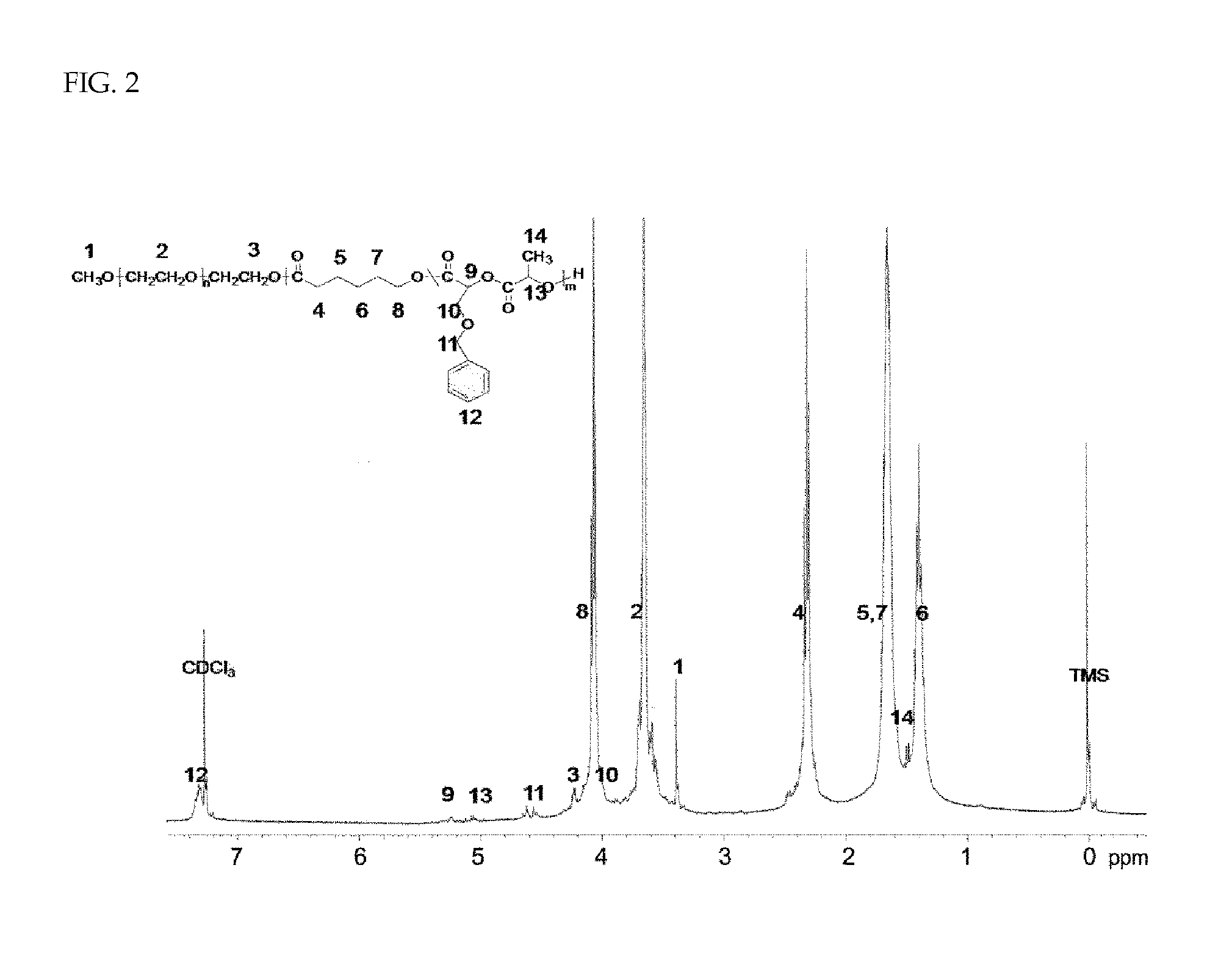
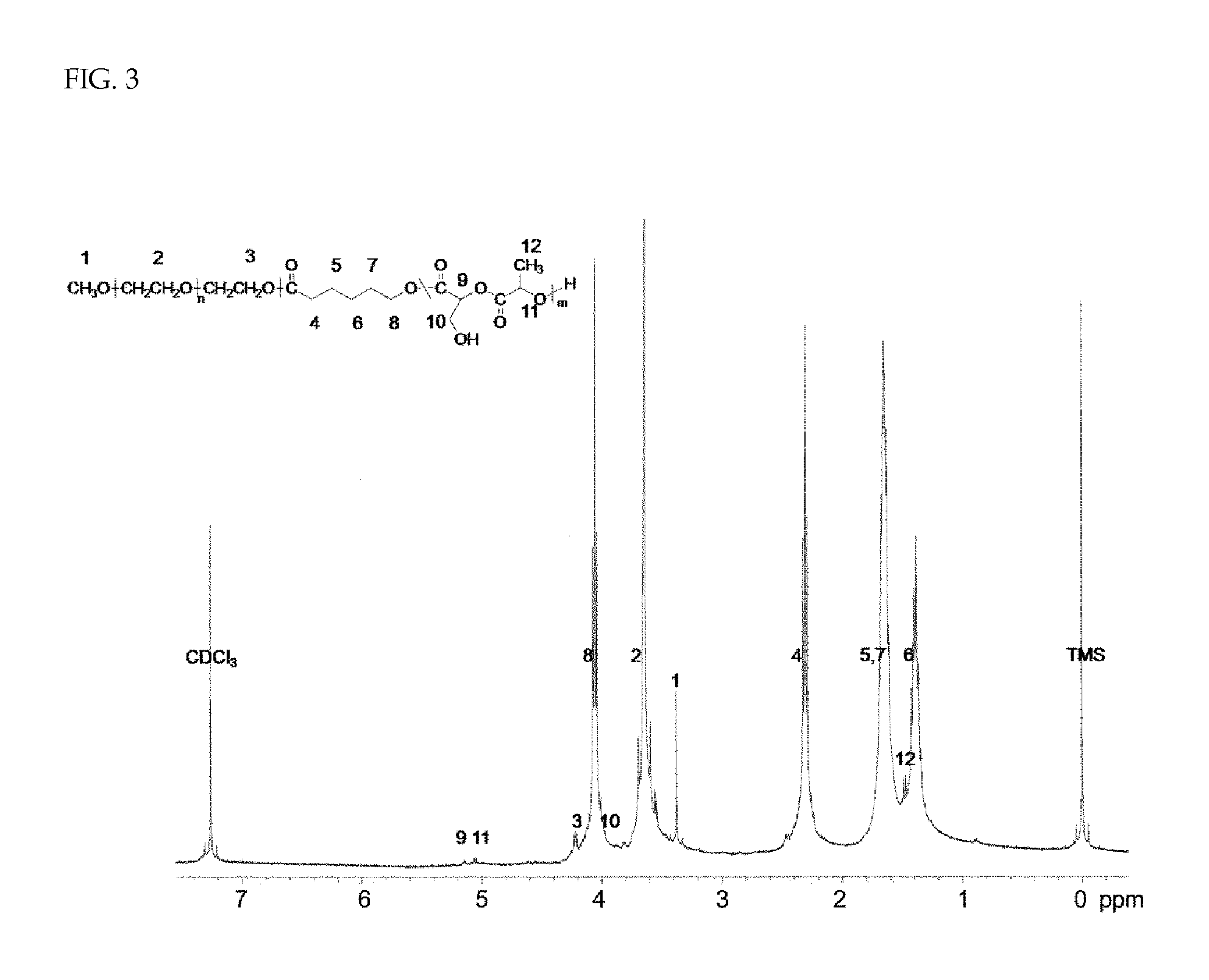
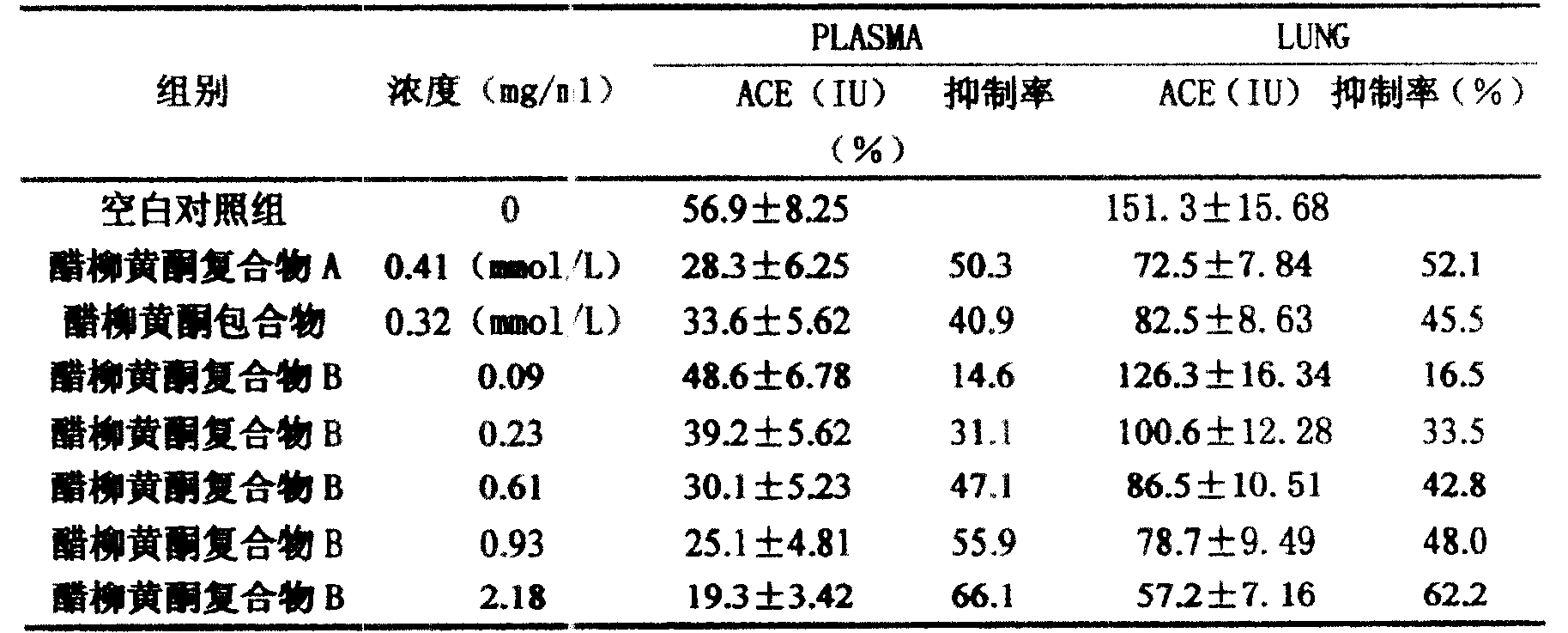
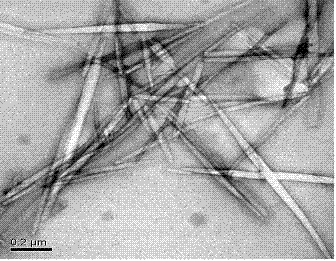
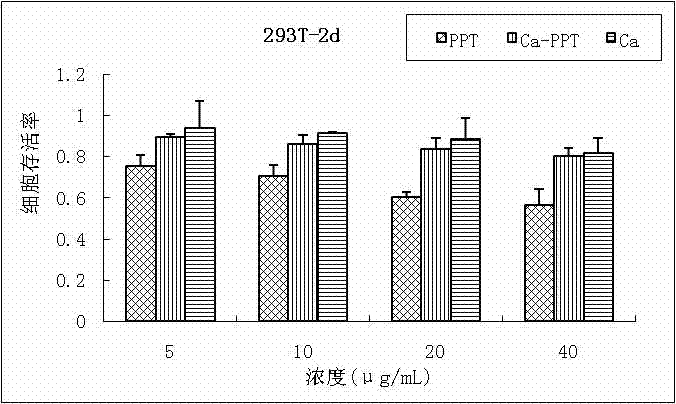
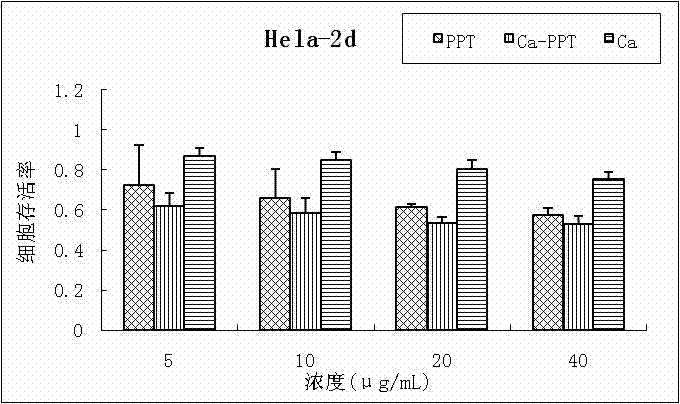
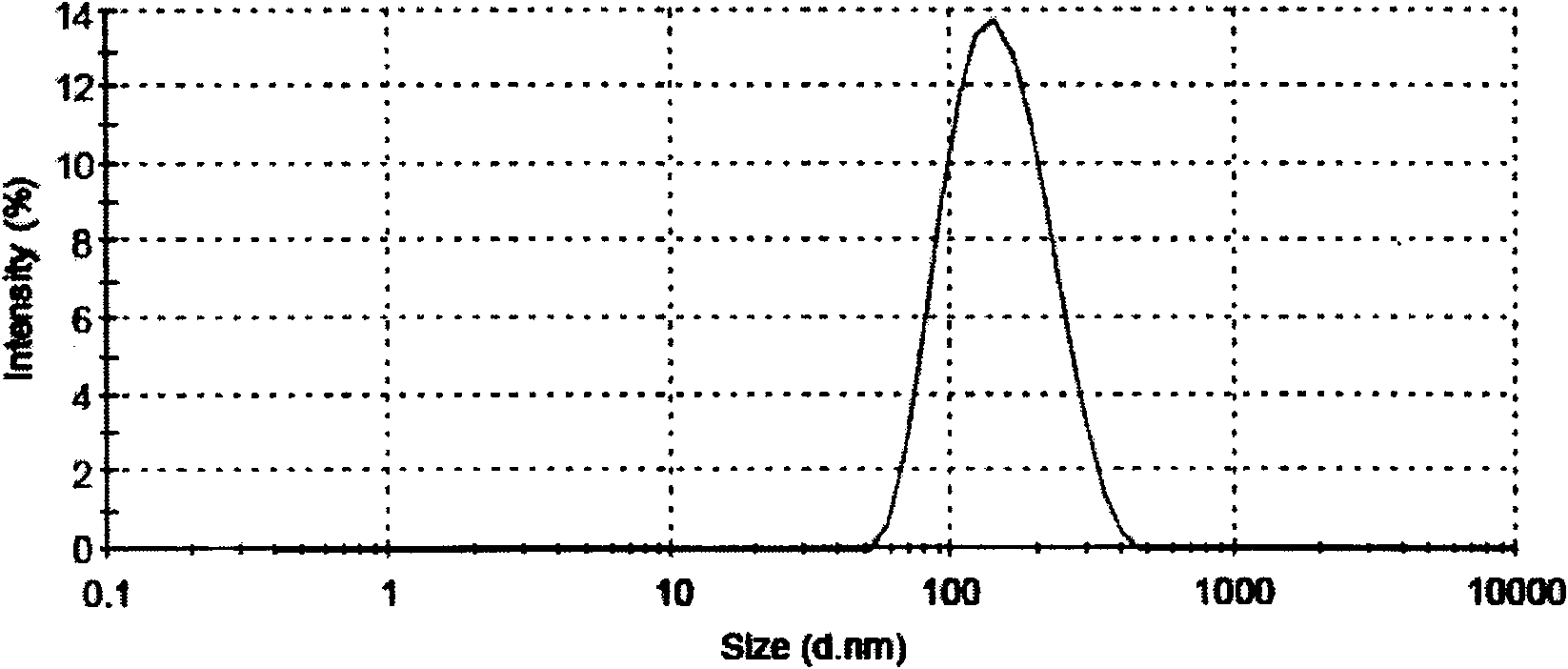
Patents
Literature
35results about How to "Stable drug loading" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Cabazitaxel liposome injection and preparation method thereof
InactiveCN103768018AHigh encapsulation efficiencyStable drug loadingOrganic active ingredientsPharmaceutical non-active ingredientsPh gradientCabazitaxel
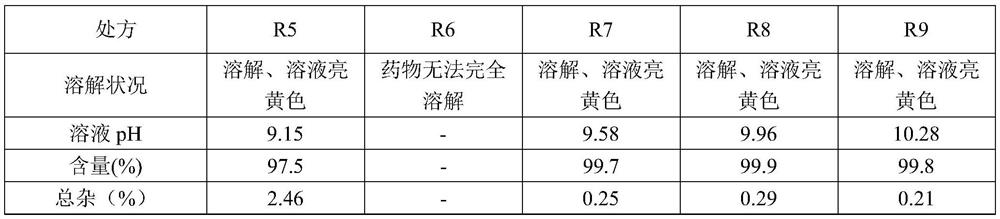
The invention provides a cabazitaxel liposome injection and a preparation method thereof. The cabazitaxel liposome injection comprises cabazitaxel, phosphatide, cholesterol, and mannitol or glucose, and can be prepared through the vacuum film condensation method, the rotary evaporation method, reverse evaporation method, high-pressure homogenization method, and pH gradient method. The cabazitaxel liposome injection has the advantages of reduced toxicity, convenience in clinic, and improved biological availability, and furthermore avoids the a plurality of changes of liposome during the storage process, such as oxidation and hydrolysis of phosphatide, agglomeration and fusion of liposome, and the like, wherein the changes can cause the leakage of coated substance. The invention provides a cabazitaxel liposome which has a good stability and is enough stable in the storage period.
Owner:NANJING LUYE PHARMA
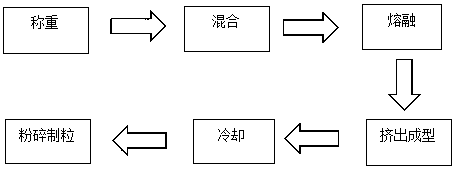
Triptorelin slow-release micro particles and preparation method thereof
ActiveCN105267153AEasy to shapeHigh encapsulation efficiencyPeptide/protein ingredientsPharmaceutical non-active ingredientsTriptorelinHot melt
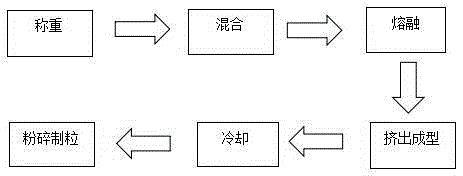
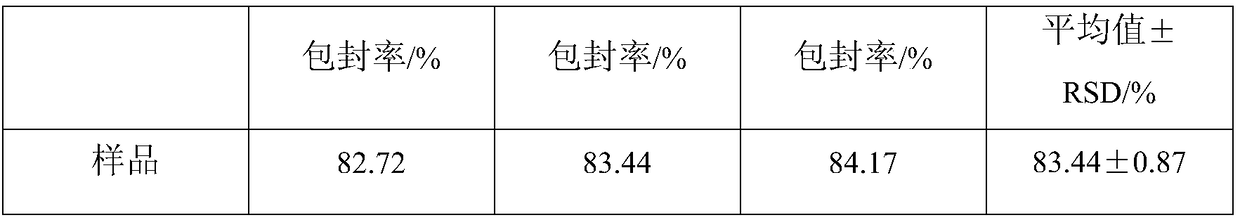
The invention relates to medicinal low-release micro particles and in particular relates to triptorelin slow-release micro particles and a preparation method thereof. The triptorelin slow-release micro particles comprise the following components in percentage by weight: 0.5-20% of triptorelin, 79-99% of PLGA and 0.1-1% of poloxamer. The preparation method of the triptorelin slow-release micro particles comprises the steps of mixing all the components, then putting the mixture into a hot melt extruder, and performing heating melting, extrusion and low-temperature crushing in the hot melt extruder. The triptorelin slow-release micro particles provided by the invention are good in shape, high in entrapment efficiency, good in drug loading capacity and stable in releasing. Moreover, the triptorelin slow-release micro particles are simple in synthetic process, are harmless, generate nontoxic products after degradation, and are stable in quality.
Owner:SHANGHAI SOHO YIMING PHARMA
Photo-thermal chemotherapy and treatment combined microenvironment responsive drug-loading nano micelle preparation method and application
ActiveCN109793710AGood anti-tumor effectDelay drug resistanceOrganic active ingredientsEnergy modified materialsPolyethylene glycolTherapeutic effect
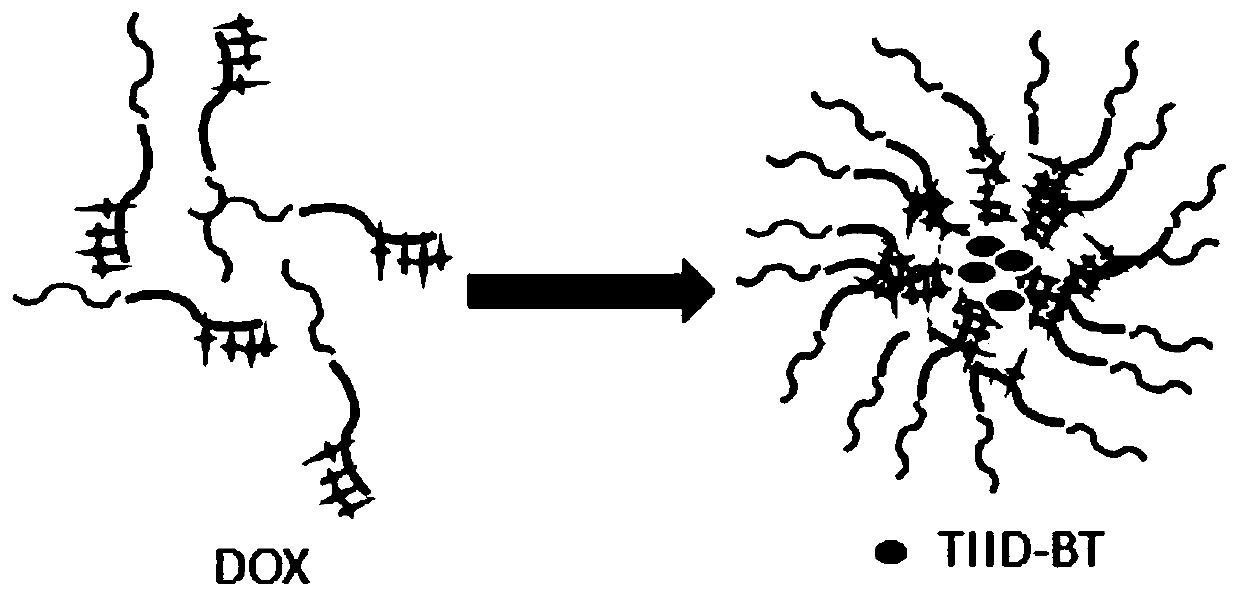
The invention discloses a photo-thermal chemotherapy and treatment combined microenvironment responsive drug-loading nano micelle preparation method and application. A nano micelle comprises TIID-BT,a medicine active component and an amphiphilic block polymer, and the amphiphilic block polymer refers to polyethylene glycol-polyaspartic acid. By synthesis, a near infrared TIID-BT dye and a DOX chemotherapy drug are loaded on the specific nano micelle at the same time to obtain the nano micelle which is capable of giving play to photo-thermal treatment and chemotherapy at the same time. The nano micelle enables DOX to avoid a combination effect of opsonin and a capturing effect of an MPS system to form a stable and long-acting drug delivery system with high drug loading capacity, and accordingly an antitumor effect of DOX is improved while DOX resistance of tumors is changed. In addition, due to loading of the TIID-BT dye in the nano micelle, in-vivo application effects of the TIID-BT dye are improved, combination of hoto-thermal treatment and chemotherapy is realized, and in-vivo tumor treatment effects are improved due to synergistic effects of DOX and TIID-BT.
Owner:SUN YAT SEN UNIV
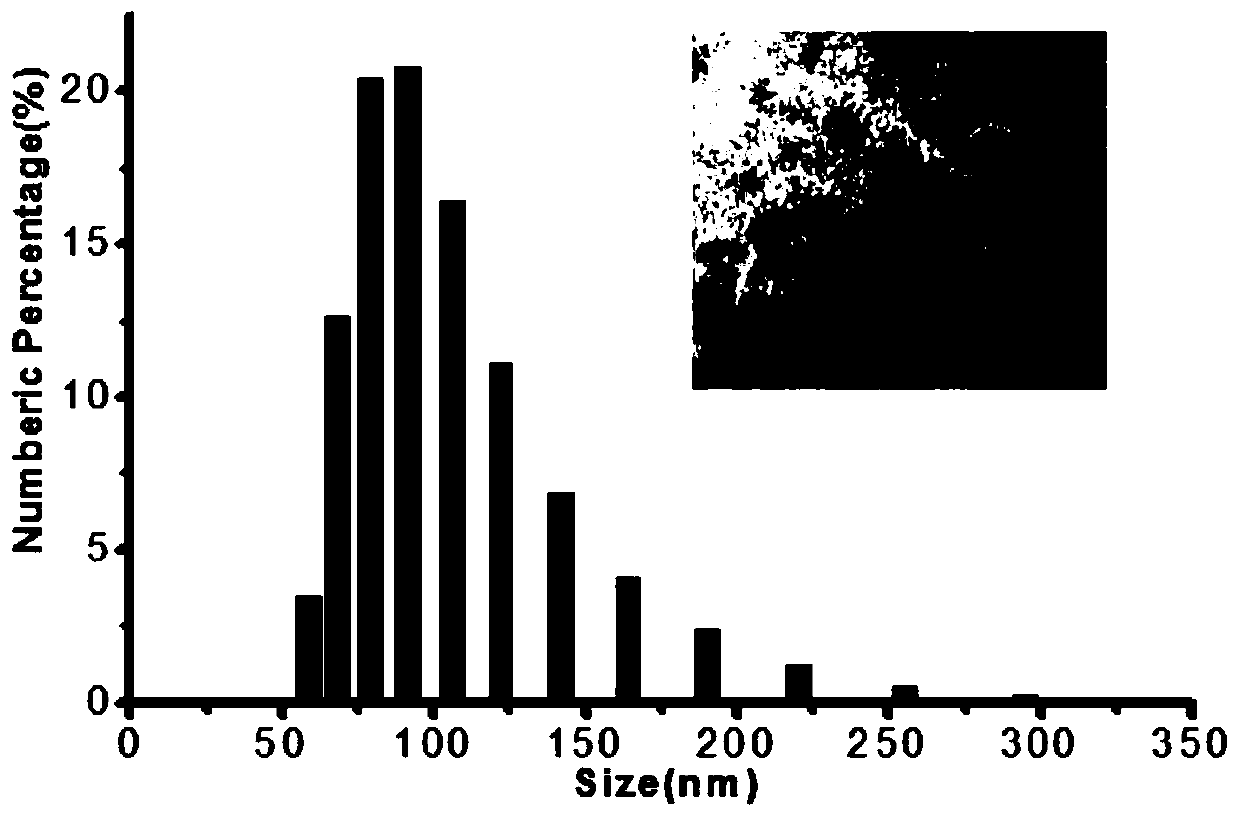
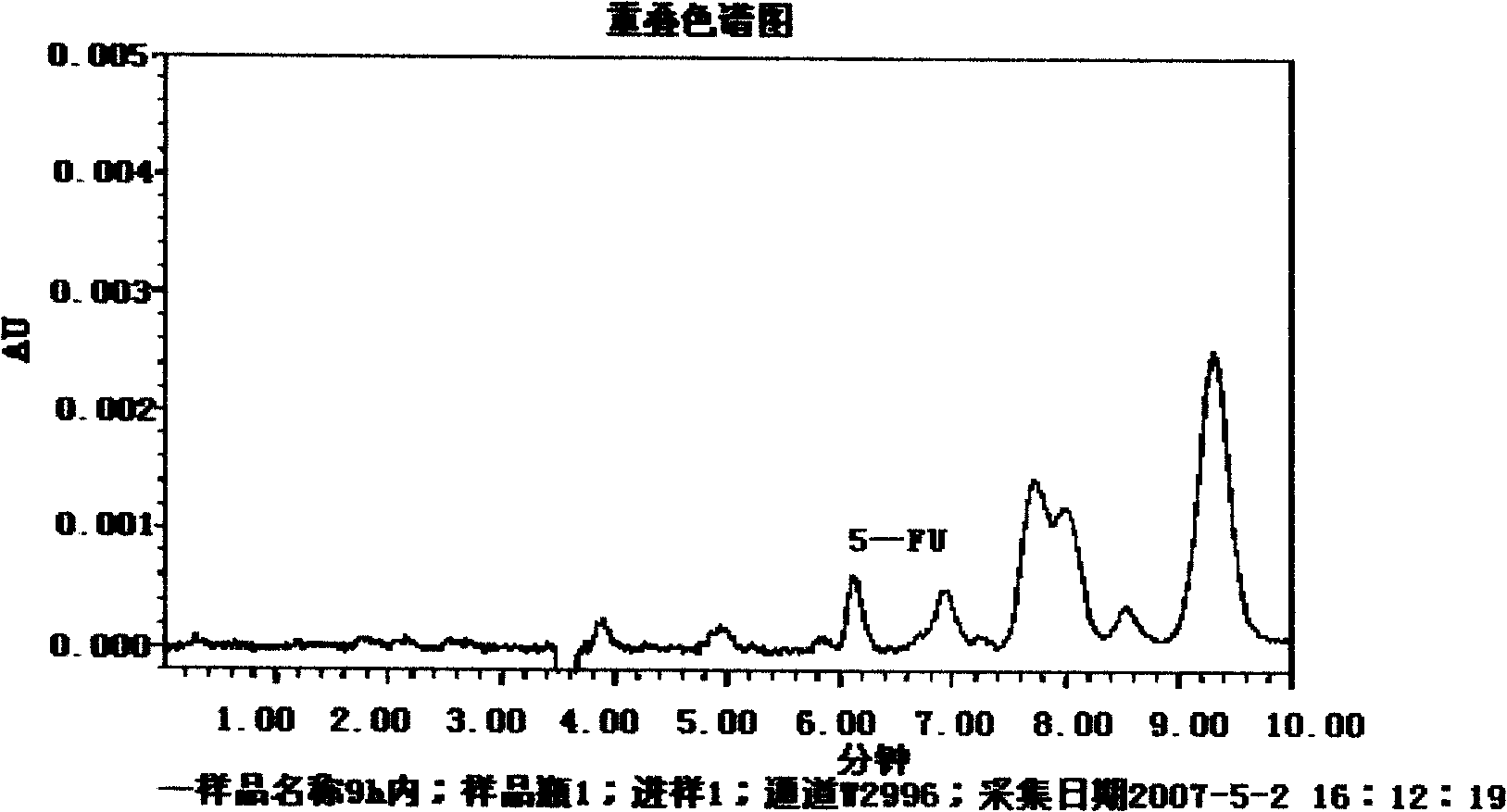
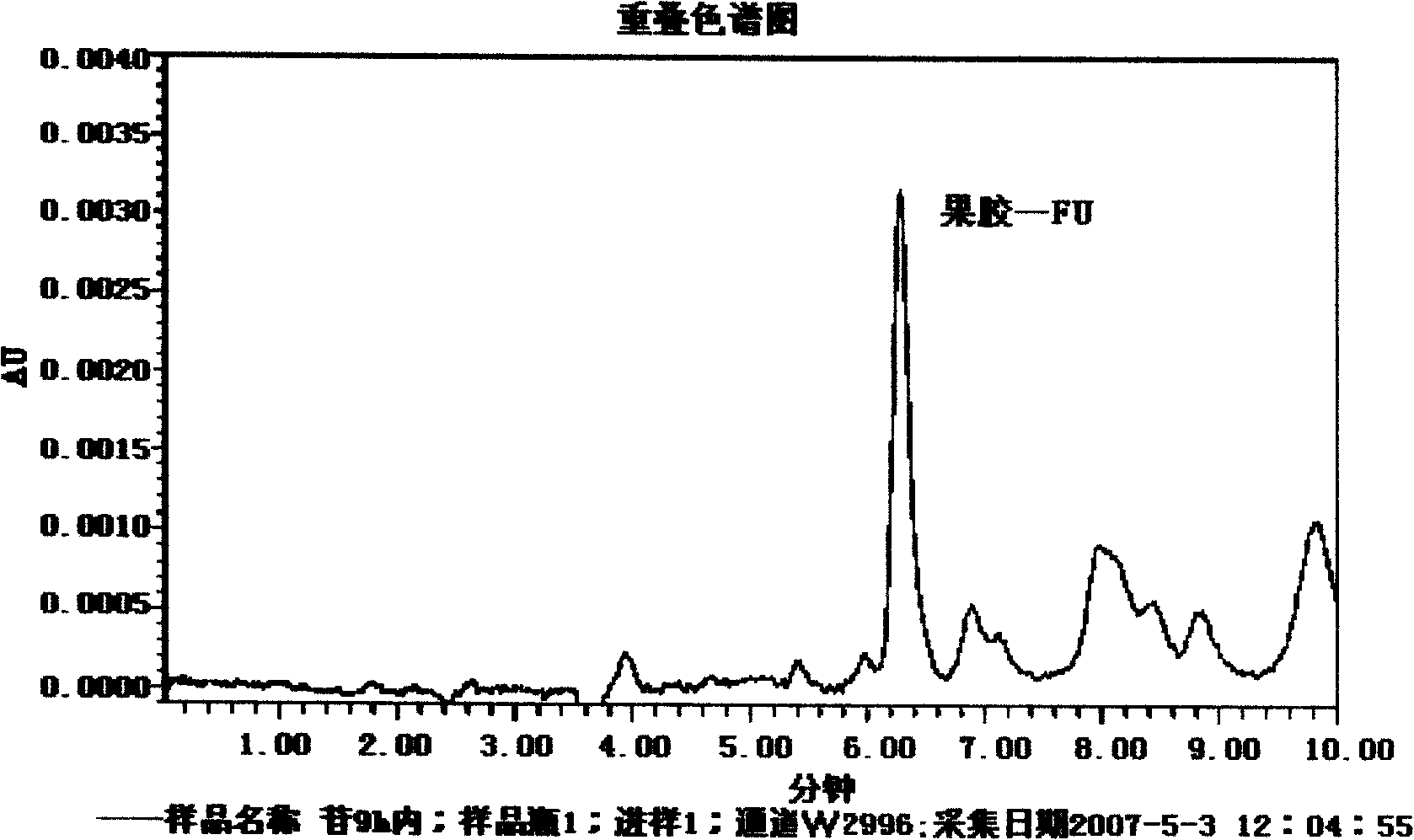
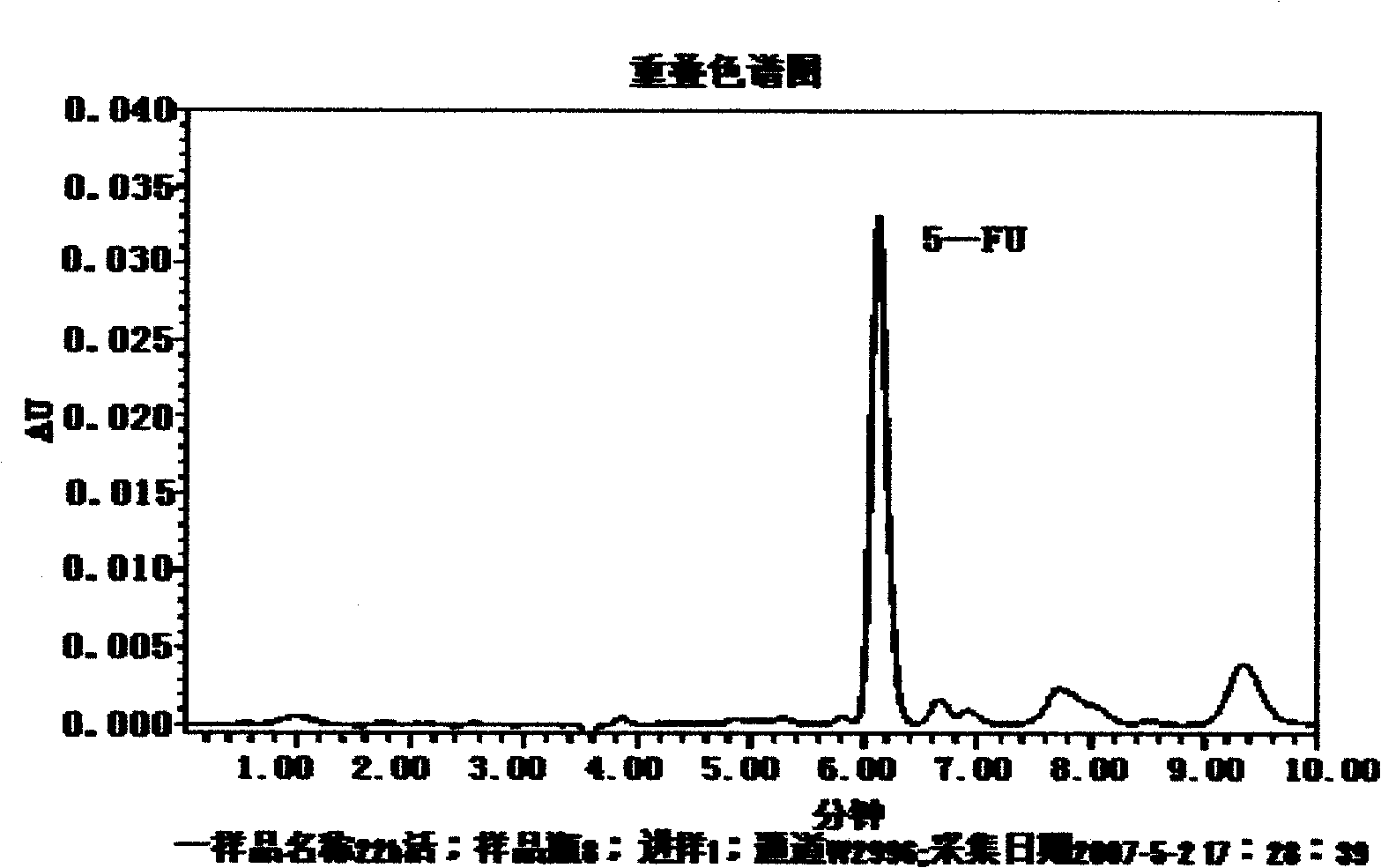
Pectin-5-efudix colon cancer double-target ahead body medicament and preparation method
InactiveCN101269087AGood treatment effectIncrease healing effect, improve selectivityOrganic active ingredientsPharmaceutical non-active ingredientsLymphatic SpreadColon cancer cell
The invention relates to a pectin-5-fluorouracil colon cancer two-targeting prodrug and the preparation method thereof, and mainly synthesizes a two-targeting prodrug for colon cancer by utilizing an anti-cancer drug, 5-fluorouracil (5-FU), and pectin. The two-targeting prodrug for colon cancer is used for treating colon cancer and is characterized in that the 6-digit carboxyl is utilized to be combined with 5-FU directly or through different bridging groups to synthesize a series of two-targeting prodrugs for colon cancer. The prodrug first targets 5-FU to the colon by utilizing pectin to realize colon positioned release, and then 5-FU-galactose is identified through high expression of colon cancer galactin-3, and then 5-Fu is targeted to the colon cancer cells to realize two-targeting of colon cancer to treat colon cancer. The prodrug improves the selectivity of 5-FU greatly, enhances the curative effect and reduces adverse reactions. In addition, the hydrolysis fragments of pectin play a role in resisting tumor metastasis and can have a synergic action with 5-FU. The pectin-5-fluorouracil two-targeting prodrug for colon cancer focuses on the drug design ideas of drug delivery, targeting and coordination; therefore, the prodrug achieves the goal of high selectivity, high efficiency and low toxicity.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
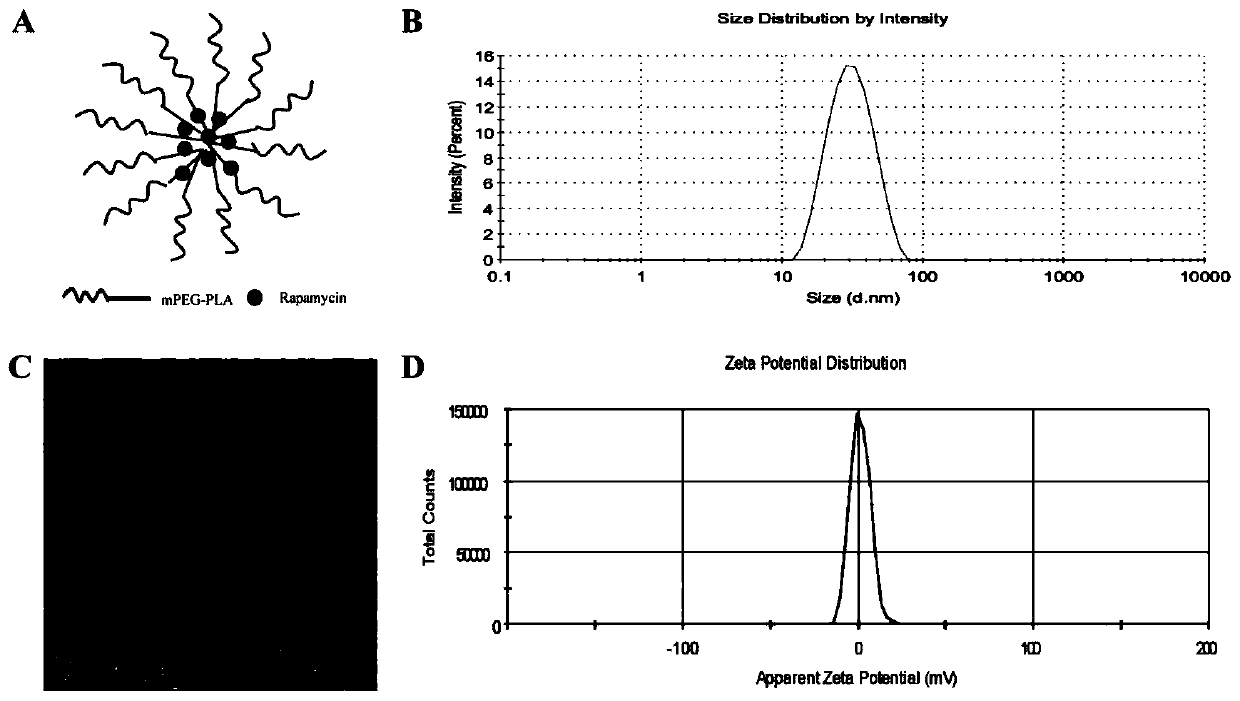
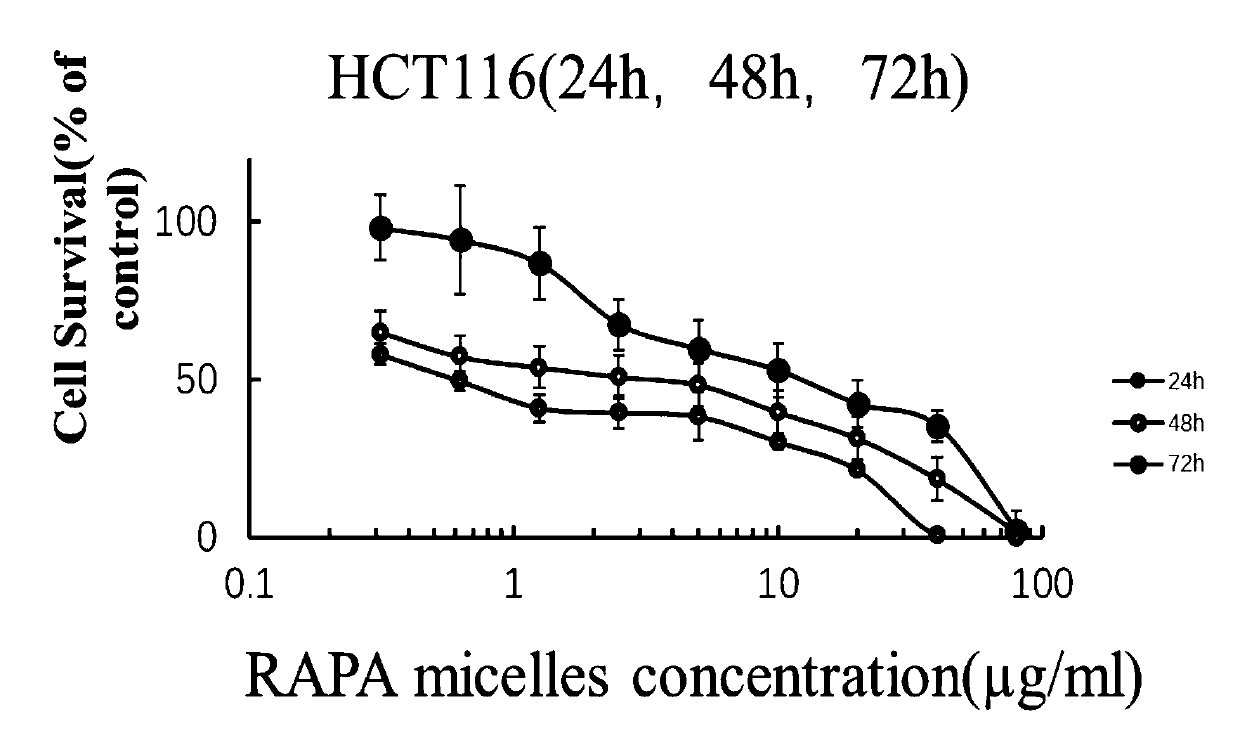
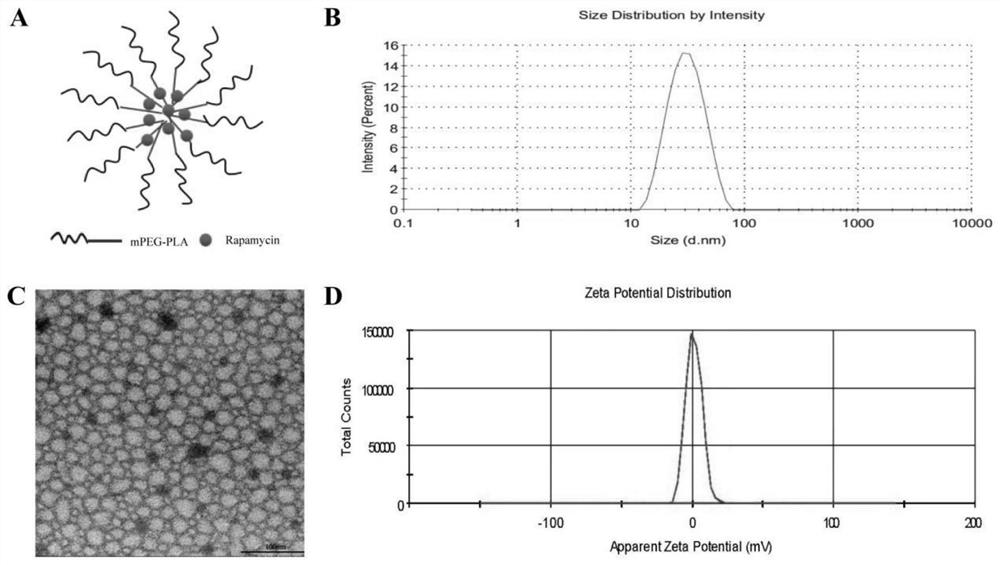
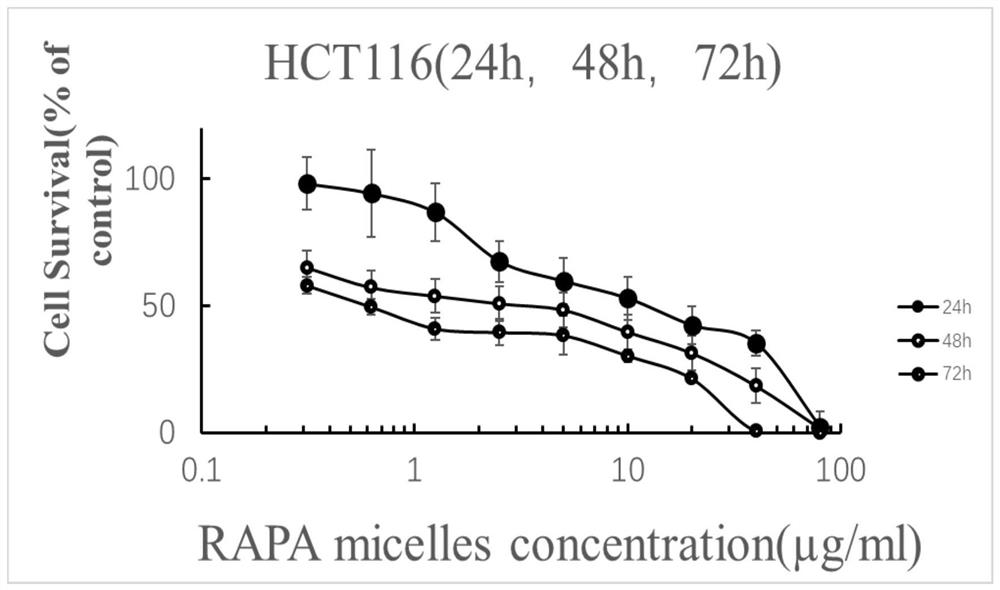
Rapamycin nano slow-release agent and preparation method thereof
ActiveCN110623925AControllable half-lifeHigh encapsulation efficiencyOrganic active ingredientsPowder deliveryOrganic solventHalf-life
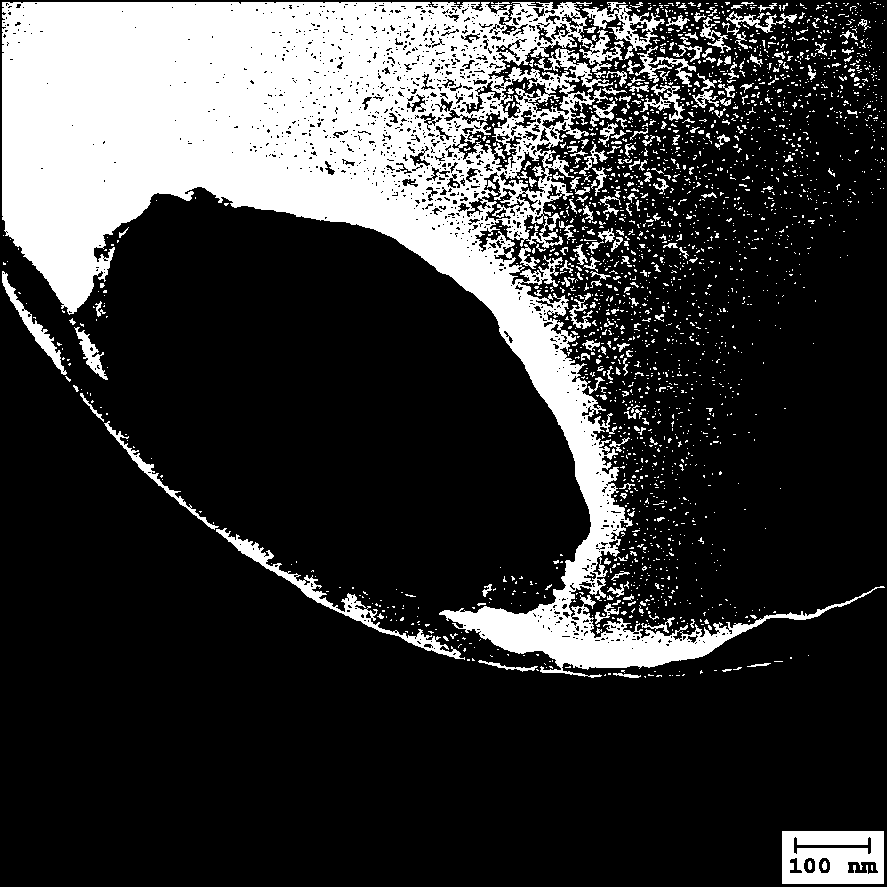
The present invention discloses a rapamycin nano slow-release agent. The rapamycin nano slow-release agent is made of the following raw materials in parts by weight: 1 part of rapamycin, 0.5-20 partsof a soluble high molecular polymer carrier, 40-200 parts of an organic solvent and 400-20,000 parts of an aqueous phase solution. The present invention also provides a preparation method of the rapamycin nano slow-release agent. The rapamycin nano slow-release agent has a nano micellar structure and particle size between 10-200 nm, and is low in risks to blood vessels. The half-life of the rapamycin nano slow-release agent in blood can be as high as 50 hours or more, the rapamycin nano slow-release agent can directly reach affected areas of tumors, is subjected to continuous administration and has a tumor regression rate of 50%.
Owner:严鹏科
Lomustine liposome freeze-drying powder injection and its preparation method
InactiveCN1839811AIncrease contentExtend cycle timePowder deliveryAmide active ingredientsEtioplastsFreeze-drying
The invention relates to an antineoplastic Lomustine liposome, its freeze-dried powder njection and preparing process, wherein the preparation comprises liposome of Lomustine and pharmaceutically acceptable carrier, the liposome of the anti-cancer Lomustine contains the following constituents: Lomustine, phosphatide, cholesterin and vitamin E, their weight ratio being 1 : 2-100 : 1-15 : 0.01-0.05.
Owner:ZHEJIANG UNIV
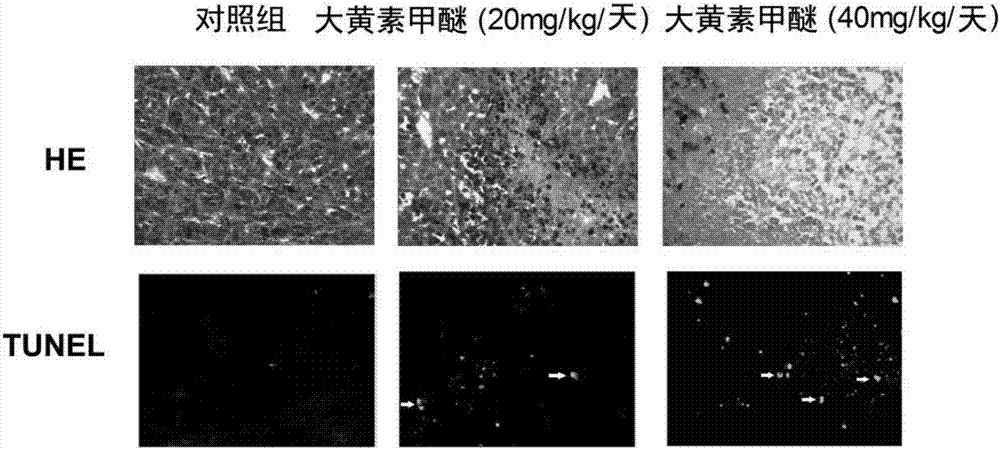
Rheochrysidin and micro-capsule of derivative of rheochrysidin as well as preparation method and application of rheochrysidin
InactiveCN107184563AConducive to exert anti-tumor effectImprove anti-tumor effectOrganic active ingredientsPharmaceutical non-active ingredientsBULK ACTIVE INGREDIENTBioavailability
The invention belongs to the field of pharmaceutical preparations, and in particular relates to rheochrysidin and a micro-capsule of a derivative of rheochrysidin as well as a preparation method and application of rheochrysidin. The invention provides an enteric-coated preparation including rheochrysidin used as an active ingredient or the derivate of the rheochrysidin, and sodium alginate-chitosan used as a capsule material. According to the rheochrysidin prepared by the invention and the enteric-coated preparation as the derivative of the rheochrysidin, the phenomenon that a conventional oral preparation is hydrolyzed and inactivated by gastric acid when the oral preparation flows through the stomach is avoided; meanwhile, by utilizing a slow release effect, the bioavailability is improved, and anti-tumor effects of the rheochrysidin and the derivate thereof are facilitated. In a preparation process of the enteric-coated preparation, a preparation technology is optimized, and the simple, feasible, safe and environment-friendly preparation method can be obtained. The multi-batch rheochrysidin and the enteric-coated preparation as the derivate of the rheochrysidin prepared according to an optimized condition are naturally dried to form white particles, and the white particles are spherical and round, uniform in size and good in dispersibility; the particle size, the drug-loading rate and the entrapment rate all keep stable, and the white particles are applied to large-scale industrial production.
Owner:潘小平
A kind of triptorelin sustained-release microparticles and preparation method thereof
ActiveCN105267153BQuality assuranceStable physical and chemical propertiesPeptide/protein ingredientsPharmaceutical non-active ingredientsMedicineTriptorelin
The invention relates to drug slow-release microparticles, in particular to triptorelin sustained-release microparticles and a preparation method thereof. The composition includes the following components by weight percentage: 0.5%-20% of triptorelin, 79%-99% of PLGA, and 0.1%-1% of poloxamer. The preparation method of the above-mentioned triptorelin sustained-release microparticles of the present invention comprises mixing each component and sending it into a hot-melt extruder, where heating and melting, extrusion and low-temperature pulverization are carried out. The praline sustained-release microparticles of the present invention have good shape, high encapsulation efficiency of the microparticles, good drug loading capacity and stable release. The synthesis process is simple, the product itself is non-toxic, and the product after degradation is non-toxic and stable in quality.
Owner:SHANGHAI SOHO YIMING PHARMA
2-methoxyestradiol lipidosome freeze-dried injection and preparation method thereof
InactiveCN101411690BHigh encapsulation efficiencyStable drug loadingOrganic active ingredientsPowder deliveryCholesterolActive agent
The invention relates to 2-methoxyl estradiol lipidosome freeze-dried powder injection and a preparation method thereof. The invention can effectively solve the problems of poor water solubility of 2-methoxyl estradiol, short half-life and low oral administration bioavailability, and adopts the technical proposal that the 2-methoxyl estradiol lipidosome freeze-dried powder injection comprises the following raw materials in weight portion: 1 portion of the 2-methoxyl estradiol, 3 to 100 portions of phospholipid, 1 to 30 portions of cholesterol, surfactant and freeze-dried preservative, wherein according to the weight ratio of the phospholipid, 0.1 to 10 portions of the freeze-dried preservative is added into 1 portion of the phospholipid, and the adding quantity of the surfactant is 0.2 to 3 percent of the total amount of the freeze-dried powder injection. The freeze-dried powder injection using the components as raw materials can be realized by three preparation methods, namely, a film dispersion method, a reverse phase evaporation method and an injection method, has high medicament content, is suitable for intravenous administration, has the advantages of slow release capability, in vivo targeting property and the like, can overcome the defects of oral preparations, is safe and reliable, and has large economic benefit and social benefit.
Owner:ZHENGZHOU UNIV
Cardiovascular pharmaceutical formulation, preparation method and uses
InactiveCN1839821AStable drug loadingLong term storageOrganic active ingredientsPowder deliverySolventDrug
Disclosed is a preparation for treating cardiovascular and cerebrovascular diseases, its preparing process and use, wherein the preparation comprises hippophe flavone, pharmaceutically acceptable inorganic bases and organic bases, or water-soluble inclusion compound formed from hippophe flavone and cyclodextrin, or low-molecular-weight alcohols, polysorbate, polyethylene glycol and poloxamer. The use of the hippophe flavone compound and preparation is also disclosed.
Owner:刘力
Method for preparing multifunctional sodium alginate stent embedded with drug-loaded microspheres by using 3D printing technology based on in-situ emulsification
ActiveCN113368304AStable drug loadingGood compatibilityAdditive manufacturing apparatusTissue regenerationMicrosphereAntiinflammatory drug
The invention discloses a method for preparing a multifunctional sodium alginate stent embedded with drug-loaded microspheres by using a 3D printing technology based on in-situ emulsification, and aims to provide a preparation method of a multifunctional bone defect repair stent material. The method is characterized by comprising the following steps: by taking a bioactive substance lecithin as an emulsifier, dispersing an amination modified polylactic acid solution dissolved with an antibacterial or anti-inflammatory drug into a sodium alginate solution to form a stable emulsion, then constructing the sodium alginate stent embedded with the drug-loaded microspheres in situ by utilizing a low-temperature 3D printing technology, and using divalent strontium ions (Sr<2+>) as a cross-linking agent to improve the mechanical properties and osteogenic activity of the stent. The method has the characteristics that the prepared tent can be individually designed according to the characteristics of the bone defect part of a patient, and the prepared stent has multiple functions of good biological activity, osteogenic ability, mechanical property, antisepsis, anti-inflammation and the like, and has potential application prospects in the field of bone tissue engineering.
Owner:FUJIAN NORMAL UNIV
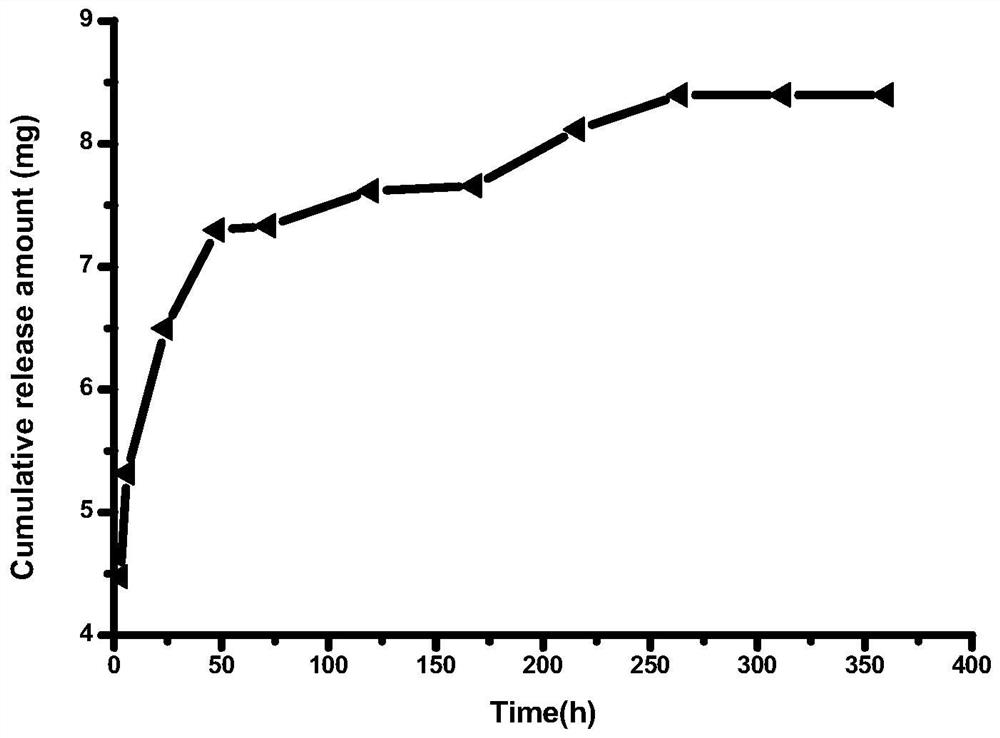
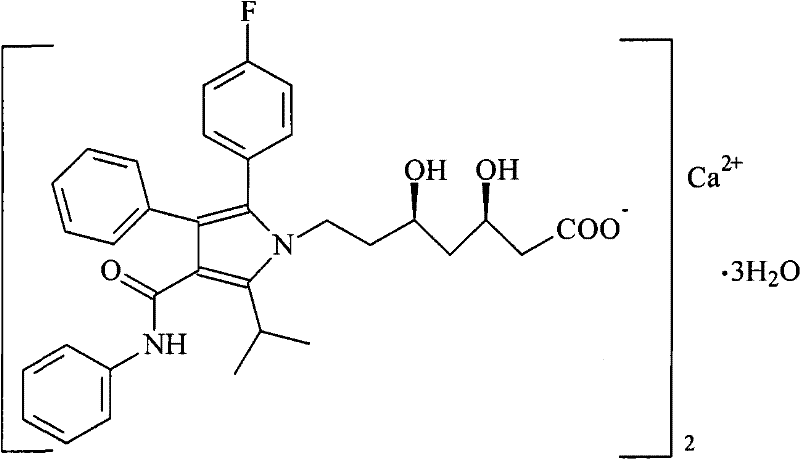
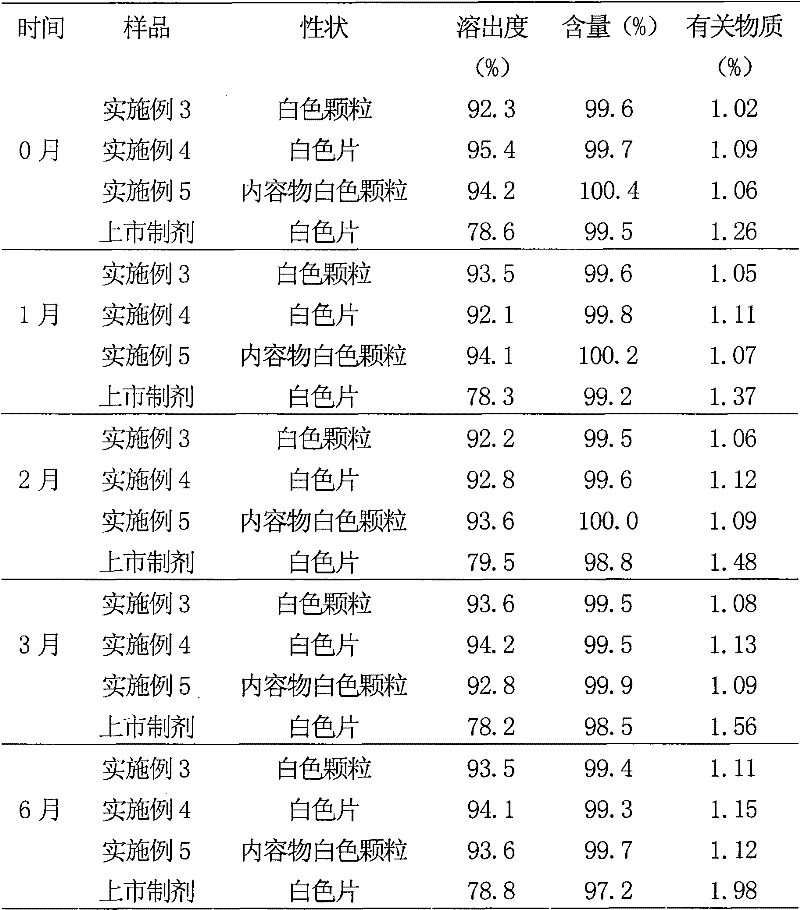
Solid preparation of atorvastatin calcium liposome
InactiveCN102008440BImprove stabilityGuaranteed efficacyMetabolism disorderPill deliveryPhospholipidBioavailability
The invention discloses a solid preparation of atorvastatin calcium liposome. The preparation is prepared from the atorvastatin calcium liposome and other pharmaceutically common auxiliary materials, wherein the atorvastatin liposome is prepared from the following components in parts by weight: 1 part of atorvastatin calcium, 2-5 parts of phospholipid and 0.8-3 parts of additive. The atorvastatinsolid preparation prepared from the liposome has the advantages that the dissolution rate is increased, the stability and bioavailability are greatly improved, the product quality of the preparation is improved and the toxic and side effect is reduced.
Owner:HAINAN MEIDA PHARMA
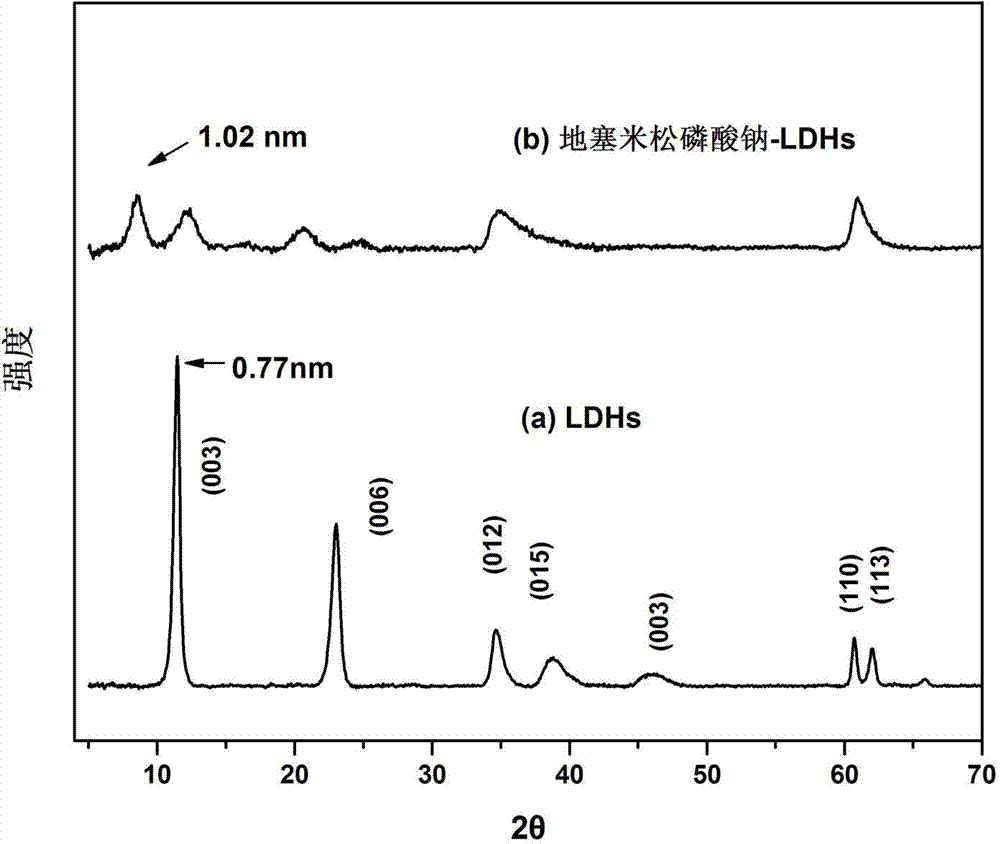
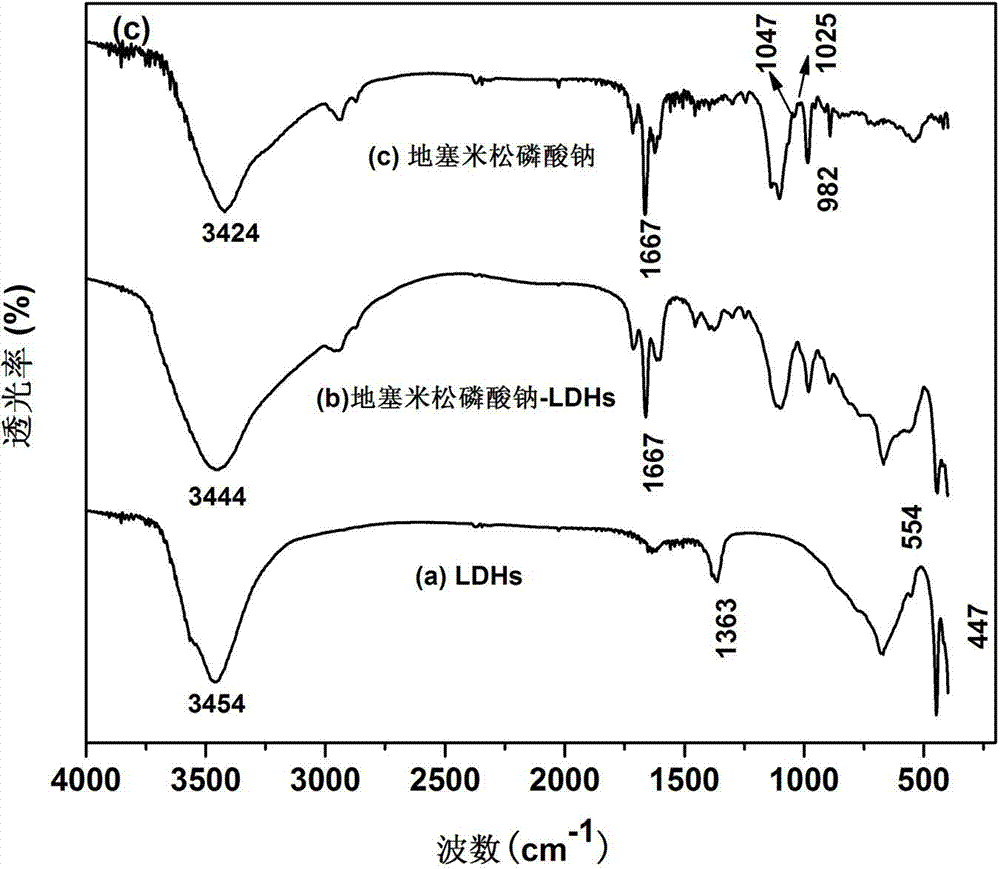
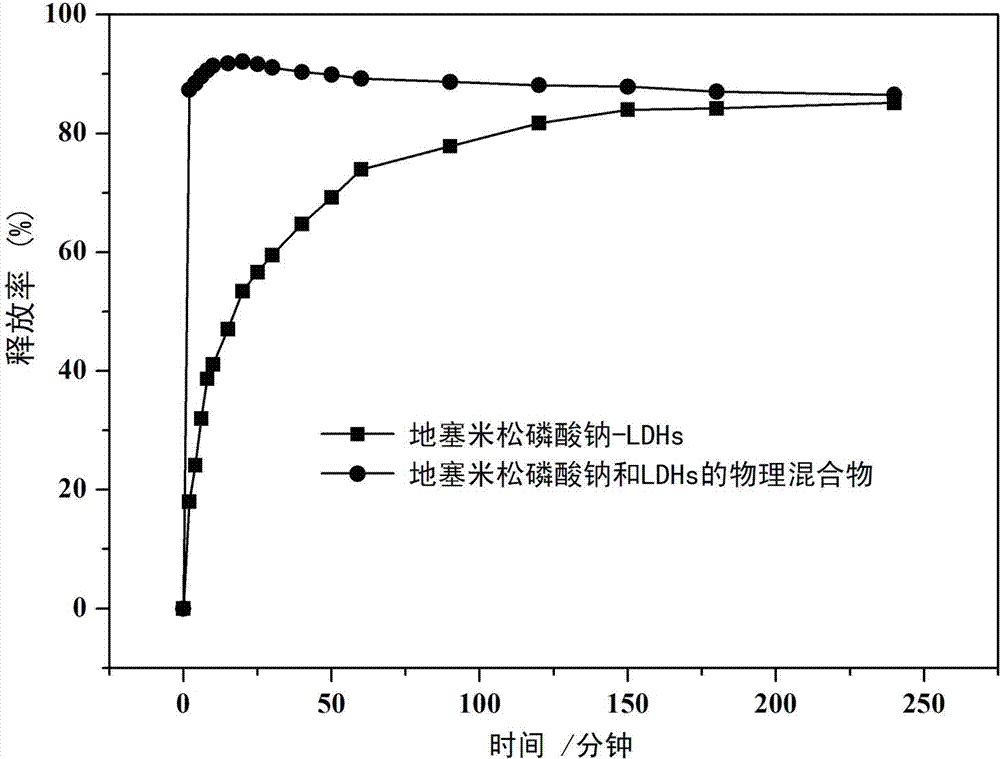
Application of dexamethasone sodium phosphate/layered double hydroxides as medicine for treating asthma and preparation method of dexamethasone sodium phosphate/layered double hydroxides
InactiveCN104274838AEasy to handleMild experimental conditionsOrganic active ingredientsPharmaceutical non-active ingredientsDiseaseSide effect
The invention belongs to the technical field of a biomedical material, and in particular relates to an application of dexamethasone sodium phosphate / layered double hydroxides in the aspect of treating asthma and a preparation method of the dexamethasone sodium phosphate / layered double hydroxides. For shortcomings of dexamethasone sodium phosphate, as a conventional glucocorticoid medicine, which is high in toxicity and poor in targeting, the dexamethasone sodium phosphate is loaded by layered double hydroxides, so as to improve a curative effect of the dexamethasone sodium phosphate and reduce toxic and side effects of the dexamethasone sodium phosphate. The prepared dexamethasone sodium phosphate / layered double hydroxides is relatively high in drug loading capacity and has a certain sustained-release effect; and the dexamethasone sodium phosphate / layered double hydroxides can inhibit egg albumin induced airway inflammation in asthmatic rats, and is expected to be applied to treatment of asthma diseases.
Owner:TONGJI UNIV
Oroxylin injection and application thereof to preparation of liver cancer drugs
ActiveCN112754991AStable drug loadingHigh drug loadingOrganic active ingredientsPharmaceutical delivery mechanismPolythylene glycolEthylene glycol
The invention discloses an oroxylin injection and an application thereof to preparation of liver cancer drugs, and belongs to the technical field of drugs. The oroxylin injection preparation is prepared from the following components: oroxylin, arginine, polyethylene glycol 400, glucose and water for injection. According to the oroxylin injection disclosed by the invention, a prescription composition and dosage cooperating with the oroxylin injection are designed and optimized from the perspective of pharmaceutics according to the physicochemical properties of the oroxylin, so that the safe and stable oroxylin injection with large drug loading capacity is obtained. The prepared oroxylin injection is used for performing pharmacodynamic investigation on human liver cancer HepG2 nude mouse xenotransplantation tumors, and a foundation is laid for clinical application of the oroxylin to treatment of liver cancer.
Owner:南京芩领医药科技有限公司
Preparation method for baicalein drug-loading nanorod
ActiveCN109432012AImprove adhesionReduce releaseAntibacterial agentsPowder deliveryWater bathsPharmacy
The invention relates to a preparation method for a baicalein drug-loading nanorod, and belongs to the field of pharmacy. The method comprises the following steps that firstly, the baicalein and pectin are weight separately and are added into a glass container, then a phosphate buffer solution is added, the glass container is immersed in a thermostatic water bath at the temperature of 50 DEG C, and magnetic stirring and uniform mixing are carried out so as to obtain a mixed solution I; then a Ca(OH)-water solution is dropwise added into the mixed solution I, and then heating is carried out for1 hour at the temperature of 50 DEG C; then a NaHCO3-water solution is dropwise added, and then heating is carried out for 3 hours at the temperature of 50 DEG C so as to obtain a mixed solution II;and finally, the baicalein is added into the mixed solution II, and magnetic stirring is continuously carried out for 24 hours at the temperature of 50 DEG C, cooling is carried out, then the mixtureis put into a dialysis bag, and dialysis is carried out in the phosphate buffer solution at the temperature of 20 DEG C for 24 hours so as to obtain the baicalein drug-loading nanorod. The baicalein drug-loading nanorod prepared through the preparation method shows good targeting property, histocompatibility, low toxic and side effects and slow-release function.
Owner:HENAN UNIV OF SCI & TECH
Drug sustained-release composition and preparation method thereof
ActiveCN110859811AImprove performanceGood particle stabilityPowder deliveryPeptide/protein ingredientsMannitolParticle composition
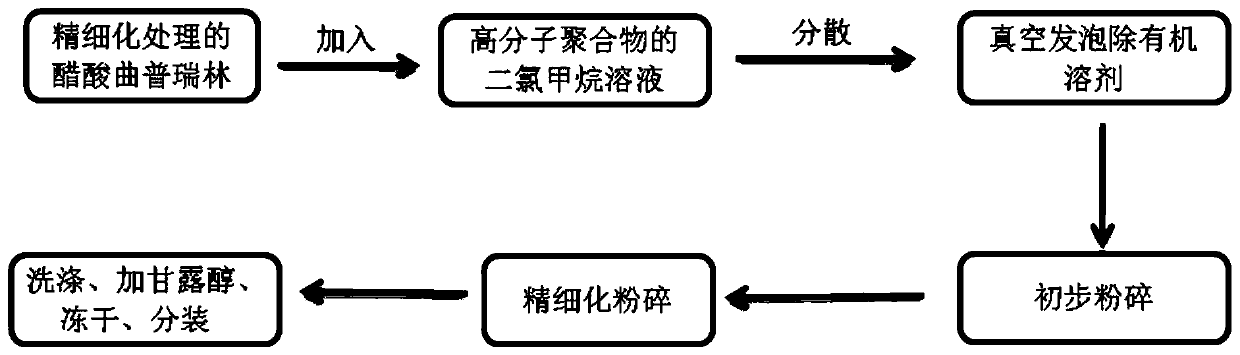
The invention discloses a drug sustained-release composition and a preparation method thereof. The drug sustained-release composition is a particle composition obtained by adopting foaming and airflowcrushing processes, and comprises 60-80 parts by weight of a fat-soluble high-molecular polymer, 1-10 parts by weight of a hydrophilic polypeptide drug and 25-35 parts by weight of mannitol. The fat-soluble high-molecular polymer is porous solid particles, the hydrophilic polypeptide drug is embedded in the fat-soluble high-molecular polymer, and the mannitol is powdery and is mixed with the fat-soluble high-molecular polymer to form the composition.
Owner:北京博恩特药业有限公司
Magnetic nano-fiber drug carrier and preparation method thereof
InactiveCN109078010AStable drug loadingLarge drug loadEnergy modified materialsInorganic non-active ingredientsElectrospinningDrug
The invention discloses a magnetic nano-fiber drug carrier and a preparation method thereof. The nano-fiber drug carrier is prepared by the following steps: a, mixing keratin and N,N-dimethylformamideand electrostatic-spinning, to obtain hollow nano keratin fibers; b, immerging in water solution of FeCl3.6H2O, FeCl2.4H2O, and organosilane, to obtain fiber dispersion liquid; c, after vacuumizing the liquid, adding hydrogen peroxide and sodium hydroxide water solution, and forming a colloid; and d, filtering, washing, and drying, to obtain the magnetic nano-fiber drug carrier. Through forming asilicon dioxide / Fe3O4 gel network in pore channels of the hollow nano keratin fibers, the magnetic nano-fiber drug carrier has the stable drug loading performance, and is large in drug loading capacity. The prepared nano-fiber drug carrier has a magnetic feature, and is capable of effectively realizing drug orientation and targeted administration.
Owner:CHENDU NEW KELI CHEM SCI CO LTD
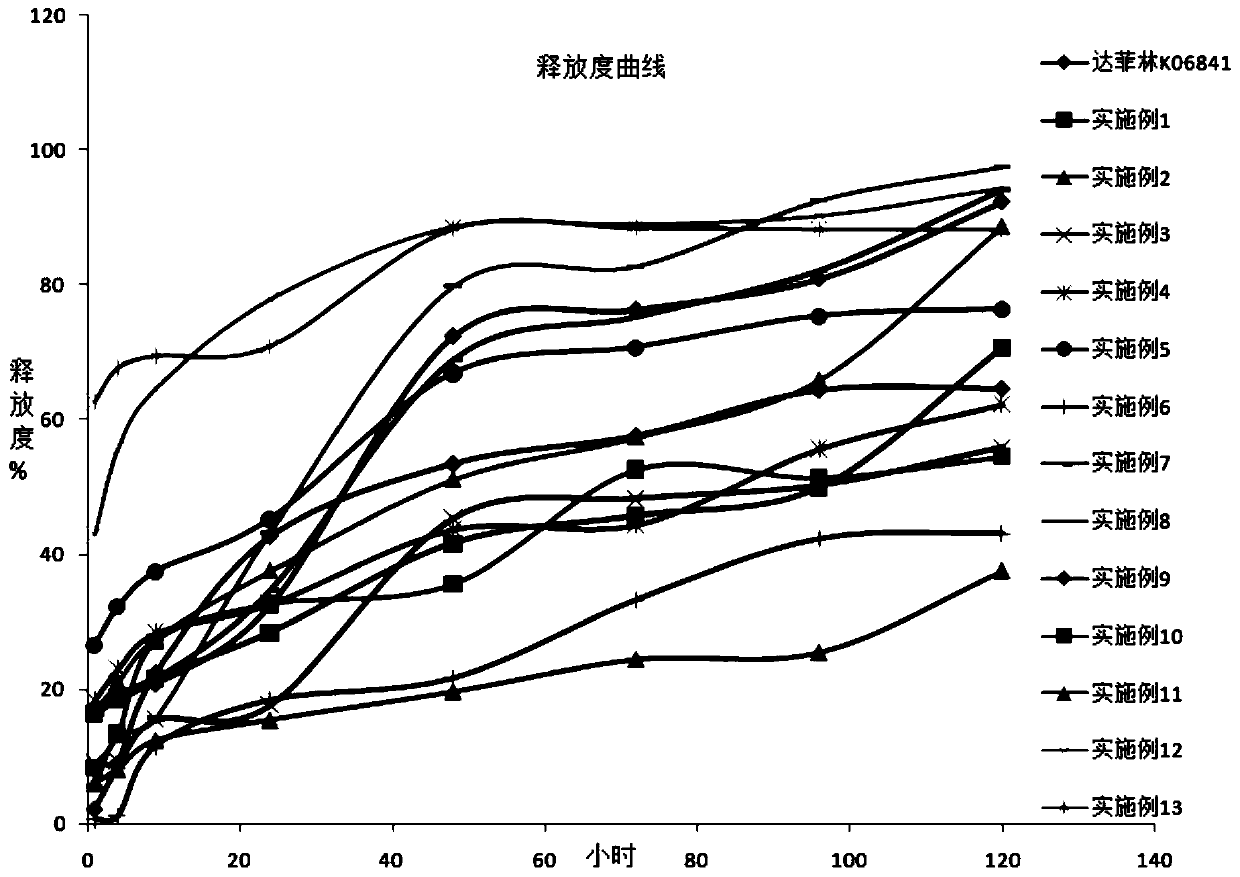
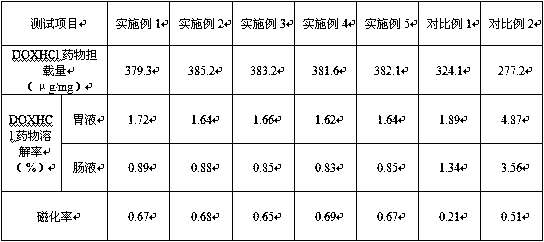
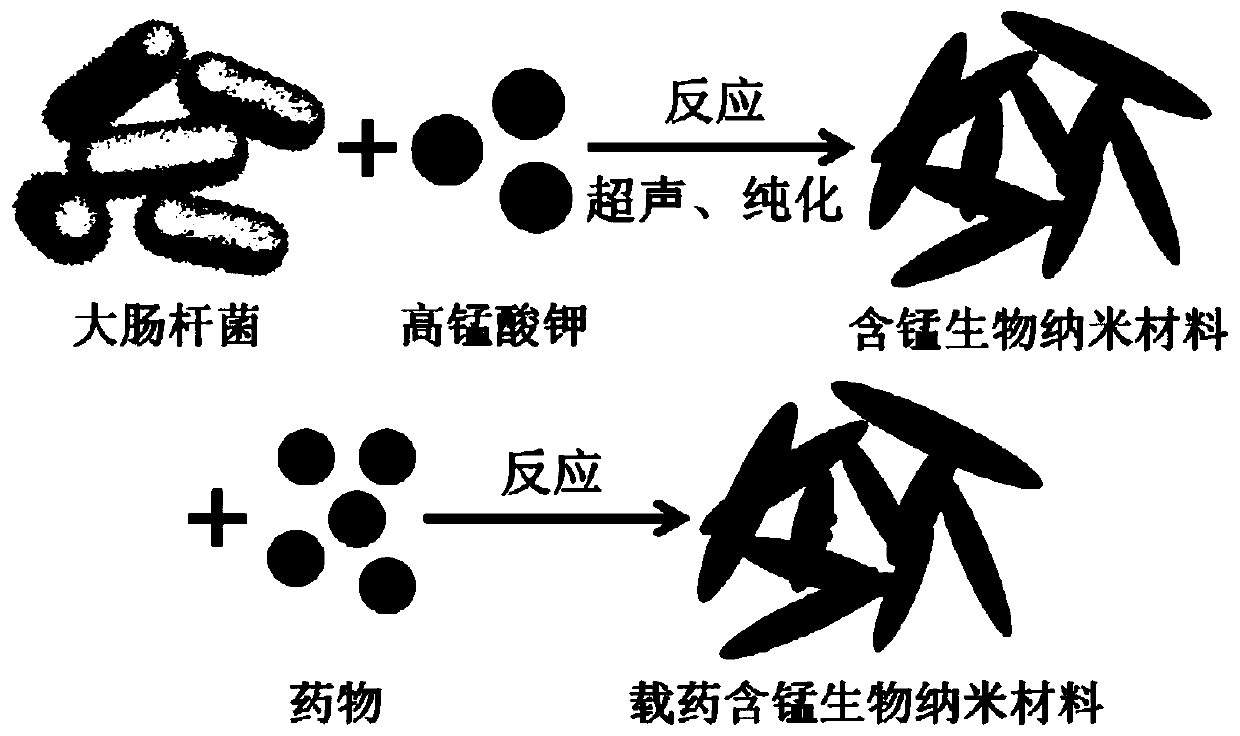
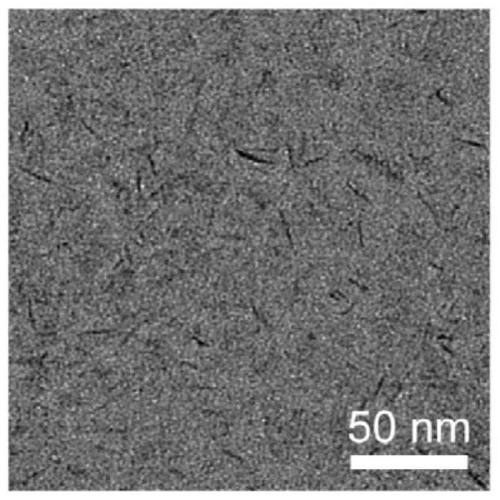
Method for synthesizing manganese-containing nano-biomaterial and application of manganese-containing nano-biomaterial
ActiveCN110343725ALow toxicityReduce photosensitivityOrganic active ingredientsPhotodynamic therapyHemolysisFluorescence
The invention discloses a method for synthesizing a manganese-containing nano-biomaterial and biomedical application of the manganese-containing nano-biomaterial. The manganese-containing nano-biomaterial is prepared from cells through potassium permanganate treatment, ultrasonic and purification. The manganese-containing nano-biomaterial has the properties of responding to glutathione and hydrogen peroxide. Furthermore, the manganese-containing nano-biomaterial can be loaded with various drugs, can be used for reducing or silencing properties of drugs, such as fluorescence, toxicity, photosensitivity, hemolysis and the like, and can controllably release an restore specific properties of the drugs under the effect of the glutathione. Therefore, the manganese-containing nano-biomaterial canbe used in catalytic oxygen generation, glutathione detection, drug delivery and the like.
Owner:SOUTHEAST UNIV
Drug sustained-release composition and preparation method
ActiveCN110859811BImprove performanceGood particle stabilityPowder deliveryPeptide/protein ingredientsMannitolPharmaceutical Substances
The invention discloses a medicine slow-release composition and a preparation method. The drug sustained-release composition of the present invention is a granular composition obtained by foaming and jet milling techniques, which comprises 60-80 parts by weight of a fat-soluble polymer, 1-10 parts by weight of a hydrophilic polypeptide drug and 25-35 parts by weight. parts by weight of mannitol. Wherein, the fat-soluble high molecular polymer is a porous solid particle, the hydrophilic polypeptide drug is embedded in the inside of the fat-soluble high molecular polymer, and the mannitol is in powder form and mixed with the fat-soluble high molecular polymer to form a composition.
Owner:北京博恩特药业有限公司
A kind of pH-sensitive nano-medicine based on calcium carbonate coordination chelation drug and its preparation method and application
ActiveCN108888600BHas acid sensitive release behaviorRelease prematurelyPowder deliveryHydroxy compound active ingredientsCarboxyl radicalAmmonium Hydrogen Carbonate
A kind of pH-sensitive nano-medicine based on calcium carbonate coordination chelation drug and its preparation method and application, calcium chloride is dissolved in absolute ethanol, then add additive solution, stir evenly, obtain solution A; Ethanol of antineoplastic drug The solution was added dropwise to solution A, and left to stand after dropping to obtain a mixed solution B; the mixed solution B and ammonium bicarbonate were placed in a vacuum oven for reaction, then dissolved in dichloromethane, and then added carboxyl-containing high Molecular substances, after stirring evenly, add 4-dimethylaminopyridine and dicyclohexylcarbodiimide, rise to room temperature, and continue to react in the dark to obtain pH-sensitive nano-medicines based on calcium carbonate coordination and chelation drugs. The preparation method of the present invention has mild conditions, easy operation, controllable size of nano medicine and good stability. The drug carrier prepared by the invention can selectively release anti-tumor drugs according to the difference in pH environment of tumor tissues and can be applied in the preparation of anti-tumor drugs.
Owner:NORTHWEST UNIV
Pectin-5-efudix colon cancer double-target prodrug and preparation method
InactiveCN101269087BEasy to synthesizeReduce synthesisOrganic active ingredientsPharmaceutical non-active ingredientsOncologyColon cancer cell
The invention relates to a pectin-5-fluorouracil colon cancer two-targeting prodrug and the preparation method thereof, and mainly synthesizes a two-targeting prodrug for colon cancer by utilizing an anti-cancer drug, 5-fluorouracil (5-FU), and pectin. The two-targeting prodrug for colon cancer is used for treating colon cancer and is characterized in that the 6-digit carboxyl is utilized to be combined with 5-FU directly or through different bridging groups to synthesize a series of two-targeting prodrugs for colon cancer. The prodrug first targets 5-FU to the colon by utilizing pectin to realize colon positioned release, and then 5-FU-galactose is identified through high expression of colon cancer galactin-3, and then 5-Fu is targeted to the colon cancer cells to realize two-targeting of colon cancer to treat colon cancer. The prodrug improves the selectivity of 5-FU greatly, enhances the curative effect and reduces adverse reactions. In addition, the hydrolysis fragments of pectin play a role in resisting tumor metastasis and can have a synergic action with 5-FU. The pectin-5-fluorouracil two-targeting prodrug for colon cancer focuses on the drug design ideas of drug delivery, targeting and coordination; therefore, the prodrug achieves the goal of high selectivity, high efficiency and low toxicity.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Temperature-sensitive polyethylene glycol / polyester block copolymer in which bioactive functional group is introduced into side chain thereof
The present invention relates to preparation and application of a temperature-sensitive polyethylene glycol / polyester block copolymer having a bioactive functional group introduced into a side chain thereof. More specifically, it relates to a temperature-sensitive polyethylene glycol / polyester block copolymer including a lactide segment having a bioactive functional group introduced into a side chain thereof and a method for preparing same. The temperature-sensitive polyethylene glycol / polyester block copolymer according to the present invention having a bioactive functional group introduced into a side chain thereof can be widely used as a drug delivery system, a support for tissue engineering, an adhesion inhibitor, etc.
Owner:MEDIPOLYMER
Cardiovascular pharmaceutical formulation preparation method and uses
InactiveCN100386076CStable drug loadingLong term storageOrganic active ingredientsPowder deliveryDiseaseAlcohol
Disclosed is a preparation for treating cardiovascular and cerebrovascular diseases, its preparing process and use, wherein the preparation comprises hippophe flavone, pharmaceutically acceptable inorganic bases and organic bases, or water-soluble inclusion compound formed from hippophe flavone and cyclodextrin, or low-molecular-weight alcohols, polysorbate, polyethylene glycol and poloxamer. The use of the hippophe flavone compound and preparation is also disclosed.
Owner:刘力
A preparation method of baicalein drug-loaded nanorods
ActiveCN109432012BImprove adhesionReduce releaseAntibacterial agentsPowder deliveryNuclear chemistryNanorod
Owner:HENAN UNIV OF SCI & TECH
Vinorelbine Bitartrate lipsome freeze-drying powder injection and its preparation method
InactiveCN100438855CExtend cycle timeSmall toxicityPowder deliveryOrganic active ingredientsFreeze-dryingAdditive ingredient
The invention relates to an antineoplastic vinorelbine bitartrate liposome, its freeze-dried powder injection and preparing process, wherein the preparation comprises liposome of vinorelbine bitartrate and pharmaceutically acceptable carrying agent, the liposome of vinorelbine bitartrate contains the following constituents: vinorelbine bitartrate, phosphatide, cholesterin and vitamin E, their weight ratio being 1 : 1-100 : 1-15 : 0.01-0.05.
Owner:ZHEJIANG UNIV +1
Method for preparing CaCO3 nanotube/podophyllum composite material
InactiveCN102240403BEvenly distributedLarge amount of productCalcium/strontium/barium carbonatesOrganic active ingredientsPhenanthrolineEngineering
The invention relates to a preparation method of a CaCO3 nanotube / podophyllum composite material, specifically: first dissolving 4.0 g of o-phenanthroline carrier in 100 ml of chloroform, vigorously stirring, and then cleaning and drying TFOM and putting it into a solution system for soaking After a certain period of time, take it out, clean it with filter paper, put it in the middle of the GRC container, and form a TFOM in the middle, and a solution phase separation system on the left and right. Prepare 0.53%-0.57% sodium carbonate solution and 2.13%-2.17% calcium chloride The solution was placed on the left and right sides of the above-mentioned TFOM respectively, and the crystal growth modifier M was added according to different situations, and the pH value was adjusted to 8. After stirring at a low speed, the solution on one side of the sodium carbonate solution was taken out, and the precipitated product was obtained by centrifugation. Water, acetone, Wash each with absolute ethanol twice, and finally store the product in absolute ethanol. The present invention has stable reaction, simple operation and easy control, low cost and no pollution, easy control of appearance and structure, uniform product distribution, not easy to agglomerate, and high purity. Easy to industrialize.
Owner:TONGJI UNIV
Chuanhuning lipid freeze-dry powder agent and preparing method thereof
InactiveCN100563649CHigh encapsulation efficiencyStable drug loadingAntibacterial agentsOrganic active ingredientsLipid formationSide effect
The invention relates to a liposome freeze-dried powder preparation containing chuanhuning and a preparation method thereof. The preparation is composed of penhuning as active ingredient, phospholipid as lipid component, emulsifier, freeze-drying protectant and auxiliary materials. Among them, the weight ratio of Chuanhuning to phospholipids is 1:1 to 200 parts, 0.0001 to 50 parts of emulsifier; 1 part of phospholipid is added with 0.1 to 30 parts of freeze-drying protective agent and appropriate amount of auxiliary materials to make a freeze-dried preparation, both The storage stability of the drug can be significantly improved, and the Chuanhuning liposome freeze-dried powder can be diluted with water for injection in any proportion and administered without precipitation and degradation products after being redispersed by adding water for injection. The encapsulation efficiency of the freeze-dried preparation is 50%-100%, and the particle size is 40nm-1000nm. The invention can be made into injections, oral preparations and pharmaceutically acceptable dosage forms, is suitable for industrial production, and has the advantages of low cost, good curative effect, few side effects, simple manufacturing process and the like.
Owner:SICHUAN UNIV
A method for preparing multifunctional sodium alginate scaffolds embedded with drug-loaded microspheres using 3D printing technology based on in-situ emulsification
ActiveCN113368304BStable drug loadingGood compatibilityAdditive manufacturing apparatusTissue regenerationMicrosphereAntiinflammatory drug
The invention discloses a method for preparing a multifunctional sodium alginate scaffold embedded with drug-loaded microspheres by using a 3D printing technology based on in-situ emulsification, and aims to provide a preparation method for a multifunctional bone defect repair scaffold material. It is characterized in that: using the biologically active substance lecithin as an emulsifier, the aminated modified polylactic acid solution dissolved in antibacterial or anti-inflammatory drugs is dispersed in a sodium alginate solution to form a stable emulsion, and then the low temperature 3D printing technology is used to in situ Sodium alginate scaffolds embedded with drug-loaded microspheres were constructed, and divalent strontium ions (Sr 2+ ) as a cross-linking agent to improve the mechanical properties and osteogenic activity of the scaffold. The feature of the present invention is that the prepared scaffold can be individually designed according to the characteristics of the patient's bone defect, and the prepared scaffold has multiple functions such as good biological activity, osteogenic ability, mechanical properties, antibacterial and anti-inflammatory, and has potential in the field of bone tissue engineering. application prospects.
Owner:FUJIAN NORMAL UNIV
A kind of ginkgo damole liposome pharmaceutical preparation and preparation method thereof
ActiveCN104971043BHigh encapsulation efficiencyStable drug loadingPharmaceutical non-active ingredientsGinkgophyta medical ingredientsGinkgo Biloba Leaf ExtractDipyridamole
The invention discloses a ginkgo damole liposome pharmaceutical preparation, which comprises 40 parts of ginkgo leaf extract, 4 parts of dipyridamole, 180-300 parts of dimyristoyl lecithin, distearoyl It is made of 60-100 parts of phosphatidylcholine, 30-50 parts of cholesterol, 20-50 parts of sucrose ester and other preparation auxiliary materials. The ginkgo damole liposome pharmaceutical preparation of the present invention can not only improve the storage stability of the pharmaceutical preparation, but also enable the sustained release of the medicine in the liposome, prolong the residence time of the medicine in the blood circulation, improve the bioavailability of the medicine, and achieve Efficient and long-acting therapeutic purposes.
Owner:GUIZHOU YIBAI PHARMA CO LTD
A kind of rapamycin nano-sustained-release agent and preparation method thereof
ActiveCN110623925BControllable half-lifeHigh encapsulation efficiencyOrganic active ingredientsPowder deliveryMedicineOrganosolv
The invention discloses a rapamycin nano-sustained-release agent, which is prepared from the following raw materials in parts by weight: 1 part of rapamycin, 0.5-20 parts of a soluble polymer carrier, and 40-200 parts of an organic solvent and 400‑20000 parts of aqueous phase. The present invention also provides a preparation method of the rapamycin nano-sustained-release agent, the rapamycin nano-sustained-release agent has a nano-micelle structure, its particle size is between 10-200nm, and has little risk to blood vessels; The half-life of pamycin nano-sustained-release agent in the blood can be as high as 50 hours or more, and it can directly reach the tumor lesion. With continuous administration, the tumor regression rate can reach 50%.
Owner:严鹏科
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com