Oroxylin injection and application thereof to preparation of liver cancer drugs
A technology of melaleucain and injection preparations, applied in the field of medicine, can solve the problems of small dosage, high solvent toxicity, poor physical stability, etc., and achieve the effect of stable drug loading
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Exploring the Prescription of Melaleucain Injection
[0024] According to the results of previous pre-prescription research, a preliminary prescription of melaleucain injection was designed, as shown in Table 1.
[0025] Table 1 Prescription of phyllothin injection
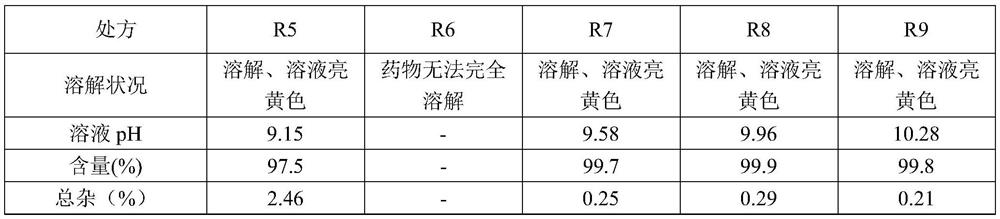
[0026] R1 R2 R3 R4 R5 R6 R7 R8 R9 Melaleuca paper 10g 10g 10g 10g 10g 10g 10g 10g 10g arginine 20g 20g 20g 20g 20g 10g 30g 40g 50g polyethylene glycol 400 0.2L 0.1L 0.0L 0.2L 0.2L 0.2L 0.2L 0.2L 0.2L glucose 50g 50g 50g 25g 0g 50g 50g 50g 50g water for injection to 1L 1L 1L 1L 1L 1L 1L 1L 1L
[0027] The dissolution status, pH, content and impurity investigation of the above-mentioned formulations were further investigated, and the specific results are shown in Table 2-1 and Table 2-2.
[0028] Table 2-1 Prescription (R1-R4) dissolution status, pH, content, impurity inspection results
[0029] prescripti...
Embodiment 2
[0035] Melaleucain Injection (1:2)
[0036] prescription:
[0037] Melaleuca paper 10g
[0038] Arginine 20g
[0039] Macrogol 400 0.2L
[0040] Glucose 50g
[0041] Water for injection to 1.0L.
[0042] Accurately weigh the prescribed amount of phyllophyllin, add the prescribed amount of polyethylene glycol 400, stir to disperse the medicine; take the prescribed amount of L-arginine and put it in another suitable container, add 50-80% of the prescribed amount of boiled Water for injection, stir to dissolve; add it to the kelp, stir to dissolve the kelp; add the prescribed amount of glucose, stir to dissolve; filter through a titanium sand core into a clean container, add Make a sufficient amount of water for injection, stir to make the solution uniform; then filter through a 0.22m microporous membrane, fill in ampoules, and seal. Sterilized by circulating steam at 100°C.
Embodiment 3
[0044] Melaleucain Injection (1:3)
[0045] prescription:
[0046] Melaleuca paper 15g
[0047] Arginine 45g
[0048] Polyethylene glycol 400 0.1L
[0049] Glucose 50g
[0050] Water for injection to 1.0L.
[0051] Accurately weigh the prescribed amount of phyllophyllin, add the prescribed amount of polyethylene glycol 400, stir to disperse the medicine; take the prescribed amount of L-arginine and put it in another suitable container, add 50-80% of the prescribed amount of boiled Water for injection, stir to dissolve; add it to the kelp, stir to dissolve the kelp; add the prescribed amount of glucose, stir to dissolve; filter through a titanium sand core into a clean container, add Make a sufficient amount of water for injection, stir to make the solution uniform; then filter through a 0.22m microporous membrane, fill in ampoules, and seal. Sterilized by circulating steam at 100°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com