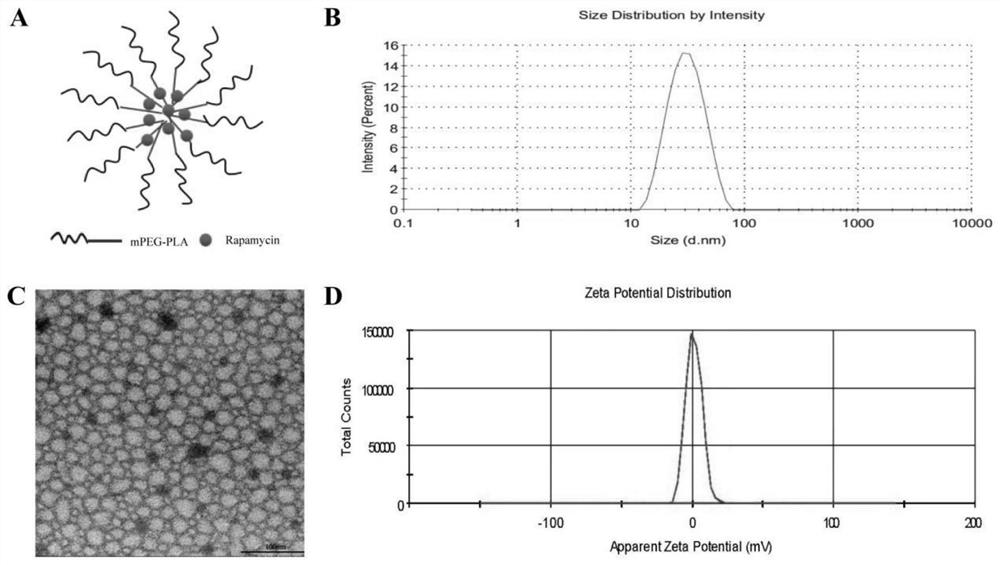
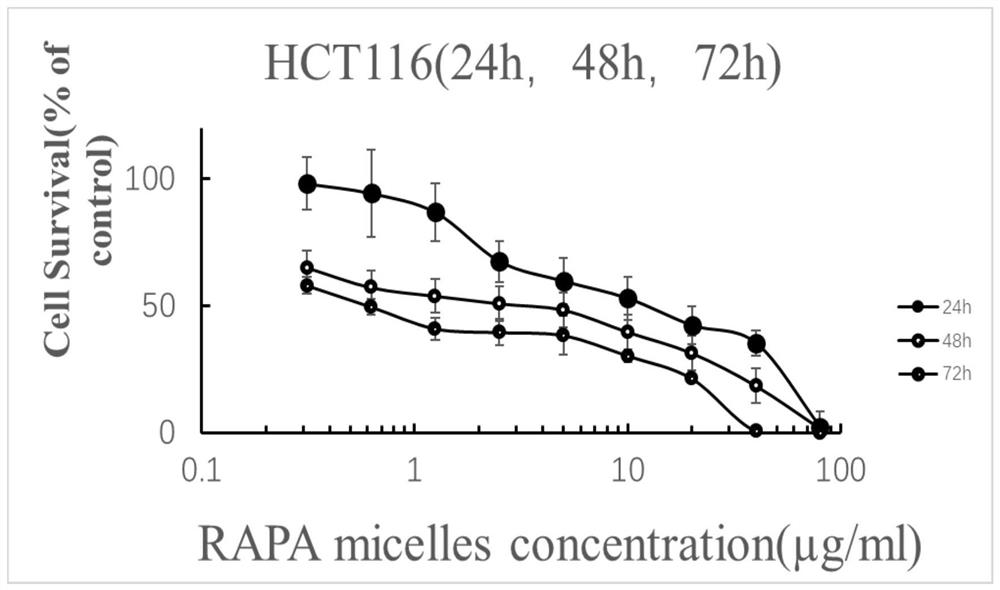
A kind of rapamycin nano-sustained-release agent and preparation method thereof
A technology for rapamycin and sustained-release agents, applied in the field of rapamycin nano-sustained-release agents and its preparation, can solve the problems of low concentration of rapamycin at the site of action, fast natural degradation rate, and insufficient targeting , to achieve stable encapsulation efficiency and drug loading, good tumor targeting, and uniform particle size distribution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0043] The preparation method of this rapamycin nano-sustained release agent, comprises the following steps:
[0044] 1) adding the rapamycin bulk drug and the soluble polymer carrier to the organic solvent to form an organic phase;
[0045] 2) Inhale the organic phase into the syringe, drop it into the aqueous phase liquid at a rate of 1-10 drops per minute, and stir at room temperature for 30min-3h;
[0046] 3) reclaiming the organic solvent under reduced pressure;
[0047] 4) Centrifuge for 5-120min, take the supernatant, and filter through a 0.22-0.45μm filter membrane to obtain a micellar solution;
[0048] 5) Freeze-drying the micellar solution to obtain the rapamycin nano-sustained release agent.
[0049] During the preparation process of the rapamycin nano-sustained-release agent, through the mutual dissolution process of the organic phase and the aqueous phase liquid, by stirring and dispersing, the micelles with small particle sizes are formed by physical force; fi...
Embodiment 1
[0053] A rapamycin nano-sustained-release agent, made of the following components: 5mg rapamycin, 10mg mPEG-PLA block polymer, 1mL acetone and 50mL PBS buffer;
[0054] Its preparation method comprises the following steps:
[0055] 1) adding rapamycin bulk drug and mPEG-PLA block polymer into acetone to form an organic phase;
[0056] 2) Inhale the organic phase into the syringe, add dropwise at a rate of 5 drops per minute to the stirring PBS buffer at 500 rpm, and stir at room temperature at 600 rpm for 60 min;
[0057] 3) Recover the organic solvent under reduced pressure at 40°C;
[0058] 4) Centrifuge at 4000r / min for 30min, take the supernatant, filter and sterilize through a microporous membrane with a pore size of 0.22μm to obtain a micellar solution;
[0059] 5) Freeze-drying the micellar solution to obtain the rapamycin nano-sustained release agent.
Embodiment 2
[0061] A rapamycin nano-sustained-release agent, made of the following components: 5mg rapamycin, 20mg mPEG-PLA block polymer, 1mL acetone and 50mL PBS buffer;
[0062] Its preparation method comprises the following steps:
[0063] 1) adding rapamycin bulk drug and mPEG-PLA block polymer into acetone to form an organic phase;
[0064] 2) Inhale the organic phase into the syringe, add dropwise at a rate of 5 drops per minute to the stirring PBS buffer at 500 rpm, and stir at room temperature at 600 rpm for 60 min;
[0065] 3) Recover the organic solvent under reduced pressure at 40°C;
[0066] 4) Centrifuge at 5000r / min for 30min, take the supernatant, filter and sterilize through a microporous membrane with a pore size of 0.22μm to obtain a micellar solution;
[0067] 5) Freeze-drying the micellar solution to obtain the rapamycin nano-sustained release agent.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com