Chuanhuning lipid freeze-dry powder agent and preparing method thereof
A technology of freeze-dried powder and shuning fat, which is applied in the field of Chuanhuning liposome freeze-dried powder preparation and preparation thereof, can solve problems such as the influence of drug stability, and achieve targeting, improved stability and improved bioavailability. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1: Preparation of Chuanhuning freeze-dried liposomes for intravenous injection
[0029] Dissolve 40mg of Chuanhuning and 400mg of soybean lecithin in an appropriate amount of acidified absolute ethanol. Large unilamellar liposomes were prepared by ethanol injection. At room temperature, inject the drug-containing ethanol solution into the buffer solution with a pH of about 3.5 under high-speed stirring. The resulting lipid solution was dried under reduced pressure at 60°C to remove ethanol and make up for evaporated water. Dissolve 50mg of Pluronic F68 in this crude liposome, and transfer to a high pressure homogenizer, under 5000Psi pressure, high pressure homogenization (Avestin) 3-8 times. The liposomes were cooled to room temperature.
[0030] Sterile filtered through a membrane filter (0.2 μm), filled into ampoules with 10% glucose as lyoprotectant, and freeze-dried.
[0031] Take the freeze-dried finished product, add appropriate amount of water for in...
Embodiment 2
[0032] Embodiment 2: the preparation of Chuanhuning liposome
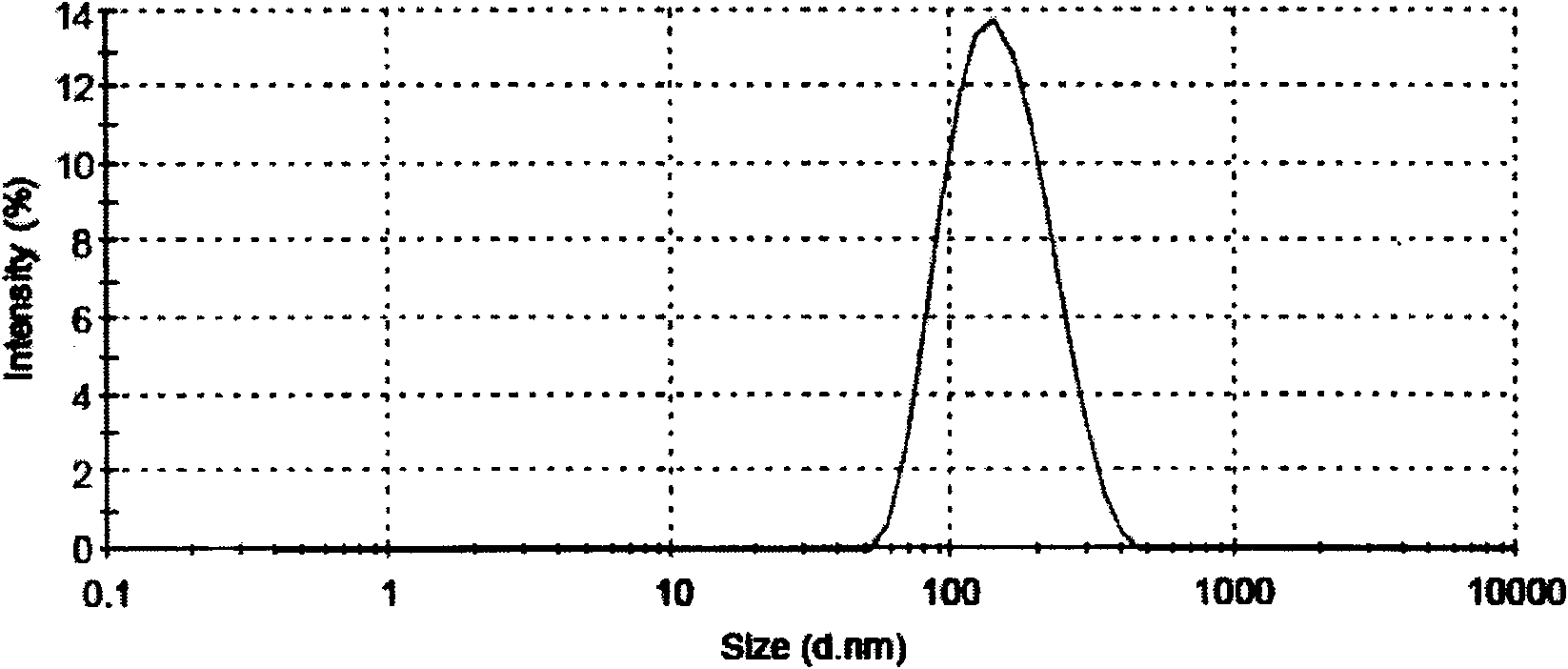
[0033] Dissolve 120 mg of penicunine and 1000 mg of synthetic phospholipid (DMPC, purity>93% phosphatidylcholine) in an appropriate amount of acidified absolute ethanol. At 60°C-70°C, mix the above drug-containing ethanol solution with Na 2 HPO 4 Aqueous solutions are mixed. The liposomes were prepared by probe ultrasonic method (150w, 10s each time, 3-10 times). The resulting lipid solution was dried under reduced pressure at 60°C to remove ethanol and make up for evaporated water. The measured encapsulation efficiency is 60%-90%, and the average particle diameter is 160nm (PDI<0.2). Diluted with 0.5% glucose solution or normal saline, no precipitation occurs within 10 hours.
Embodiment 3
[0034] Embodiment 3: Determination of encapsulation efficiency
[0035] Measured by ultrafiltration. Draw 0.1ml Chuanhuning liposome respectively, add 0.4ml pH3.5 buffer solution, 5000rpm, 15min ultrafiltration. Take 10 μl of the supernatant and inject it into the sample, and calculate the peak area A of the free penetrant 1 . Take another 0.1ml of Chuanhuning liposome, dilute to 10ml with methanol, take 10μl of sample, calculate the total Chuanhuning peak area A 0 .
[0036] Encapsulation efficiency (EE%) was calculated according to the following formula:
[0037] EE%=(1-A 1 / A 0 )×100%
[0038] The calculated encapsulation efficiency is between 80% and 99.8%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com