A kind of ginkgo damole liposome pharmaceutical preparation and preparation method thereof
A technology of pharmaceutical preparations and damolipid, applied in the field of medicine, can solve the problems of limited physical and chemical properties and pharmacokinetic characteristics, low bioavailability of preparations, and difficulty in obtaining curative effects, etc., to achieve improved bioavailability, improved therapeutic effect, The effect of prolonging the residence time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
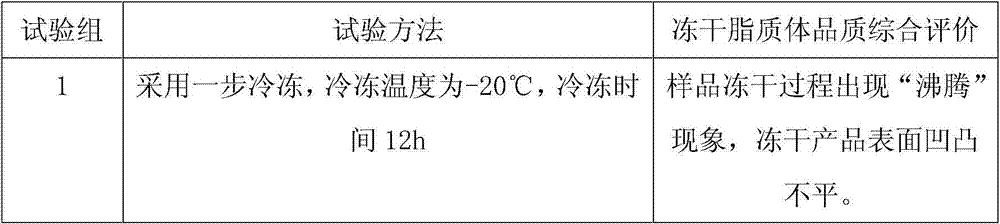
Examples
Embodiment 1
[0039] Embodiment 1 Ginkgo Damole liposome preparation
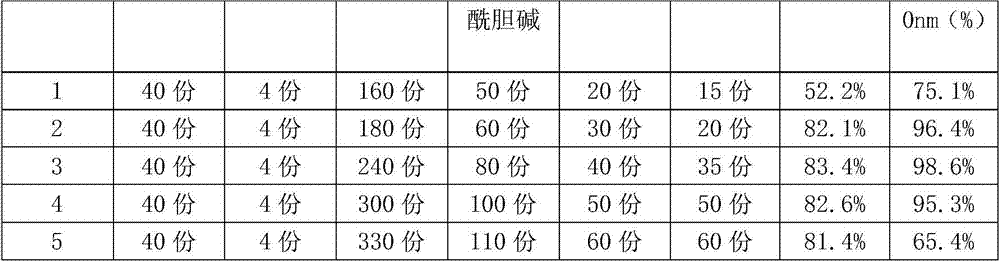
[0040] Preparation prescription: Ginkgo biloba extract 4.0g, dipyridamole 0.4g, dimyristoyl lecithin 18.0g, distearoylphosphatidylcholine 6.0g, cholesterol 3.0g, sucrose ester 2.0g;
[0041]Preparation method: Using film hydration-high pressure homogenization method, 4.0g of Ginkgo biloba extract, 0.4g of dipyridamole, 18.0g of dimyristoyl lecithin, 6.0g of distearoylphosphatidylcholine, and 3.0g of cholesterol g and sucrose ester 2.0 in an eggplant-shaped bottle, add 300ml of a 1:3 mixture of absolute ethanol-dichloromethane to dissolve it, place it in a rotary evaporation at 40°C until a film is formed, dry it under reduced pressure overnight, and add pH=6.5 PBS phosphate buffer solution was hydrated at room temperature, and the resulting milky white suspension was homogenized by a high-pressure homogenizer with a homogenization pressure of 15,000 psi, circulated 3 times, and dried to obtain Ginkgo Damole liposomes.
Embodiment 2
[0042] Embodiment 2 ginkgo damo liposome preparation
[0043] Preparation prescription: Ginkgo biloba extract 4, dipyridamole 0.4g, soybean lecithin 24.0g, distearoylphosphatidylcholine 8.0g, cholesterol 4.0g, sucrose ester 3.5g;
[0044] Preparation method: Using film hydration-high pressure homogenization method, 4.0g of Ginkgo biloba extract, 0.4g of dipyridamole, 24.0g of dimyristoyl lecithin, 8.0g of distearoylphosphatidylcholine, and 4.0g of cholesterol g and sucrose ester 3.5 are placed in an eggplant-shaped bottle, add 400ml of a 1:3 mixture of absolute ethanol-dichloromethane to dissolve it, place it in a rotary evaporation at 40°C until a film is formed, dry it under reduced pressure overnight, and add pH=6.5 PBS phosphate buffer solution was hydrated at room temperature, and the resulting milky white suspension was homogenized by a high-pressure homogenizer with a homogenization pressure of 15,000 psi, circulated 3 times, and dried to obtain Ginkgo Damole liposomes....
Embodiment 3
[0045] Embodiment 3 ginkgo damo liposome preparation
[0046] Preparation prescription: Ginkgo biloba extract: 4.0, dipyridamole: 0.4g, dimyristoyl lecithin: 30.0g, distearoylphosphatidylcholine: 10.0g, cholesterol: 5.0g, sucrose ester: 5.0g ;
[0047] Preparation method: using film hydration-high pressure homogenization method, 4.0g of ginkgo biloba extract, 0.5g of dipyridamole, 30.0g of soybean lecithin, 10.0g of distearoylphosphatidylcholine, 5.0g of cholesterol and sucrose ester Put 5.0g in an eggplant-shaped bottle, add 400ml of 1:3 absolute ethanol-dichloromethane mixture to dissolve it, put it in a rotary evaporation at 40°C to form a film, dry it under reduced pressure overnight, add PBS phosphoric acid with pH=6.5 The salt buffer solution was hydrated at room temperature, and the obtained milky white suspension was homogenized by a high-pressure homogenizer at a homogenization pressure of 15,000 psi, circulated 3 times, and dried to obtain Ginkgo Damole liposomes. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com