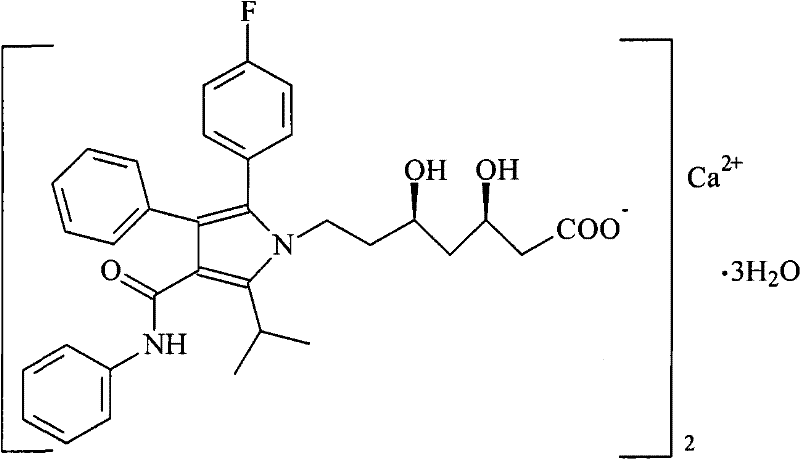
Solid preparation of atorvastatin calcium liposome
A technology of atorvastatin calcium and solid preparations, which is applied in the field of medicine, can solve the problems of poor stability and bioavailability, and cannot effectively exert the pharmacological activity of atorvastatin, and achieve the protection of vascular endothelial cells and microvascular system, The effect of reducing myocardial infarction size and stabilizing drug loading
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
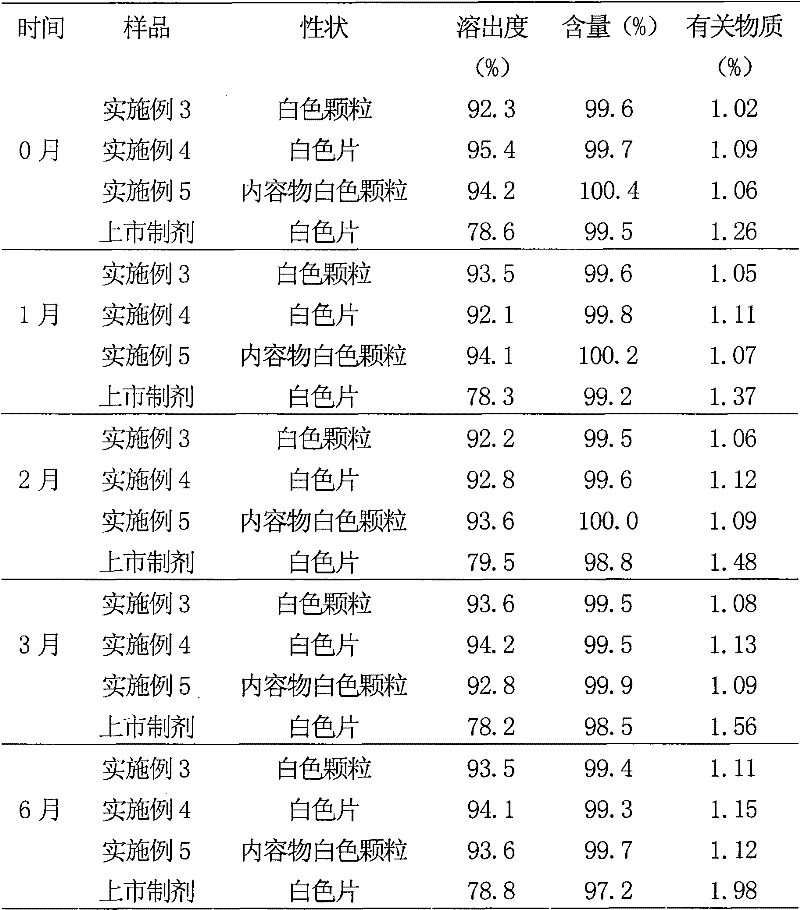
Examples
Embodiment 1
[0052] Example 1 Preparation of Atorvastatin Calcium Liposome
[0053] prescription:
[0054] Atorvastatin Calcium 100g
[0055] Soy Lecithin 133.3g
[0056] Dimyristoyl Phosphatidylethanolamine 66.7g
[0057] Cholesterol 40g
[0058] Octadecylamine 40g
[0059] Preparation Process:
[0060] (1) Dissolve 133.3g soybean lecithin, 66.7g dimyristoylphosphatidylethanolamine, 40g cholesterol and 40g octadecylamine in a mixed solvent of ethyl acetate and ethanol with a volume ratio of 3:2 in 2000ml, and place on a rotating film The organic solvent was removed under reduced pressure on the evaporator, and the phospholipid film was obtained;
[0061] (2) Add 800 ml of citric acid-sodium citrate buffer solution with 100 g of atorvastatin calcium and a pH value of 5.6 to fully hydrate the phospholipid film, mix the solution evenly, and heat it at 70° C. for 40 minutes with ultrasonic treatment;
[0062] (3) Spray-dry the solution obtained in the above step (2) to obtain atorva...
Embodiment 2
[0085] Example 2 Preparation of Atorvastatin Calcium Liposome
[0086] prescription:
[0087] Atorvastatin Calcium 100g
[0088] Soy Lecithin 333.3g
[0089] Dimyristoyl Phosphatidylethanolamine 166.7g
[0090] Cholesterol 150g
[0091] Octadecylamine 150g
[0092] Preparation Process:
[0093] (1) Dissolve 333.3g soybean lecithin and 166.7g dimyristoylphosphatidylethanolamine and 150g cholesterol and 150g octadecylamine in a mixed solvent of ethyl acetate and ethanol with a volume ratio of 3:2 in 6000ml, and place on a rotating film The organic solvent was removed under reduced pressure on the evaporator, and the phospholipid film was obtained;
[0094] (2) Add 3000 ml of citric acid-sodium citrate buffer solution with 100 g of atorvastatin calcium and a pH value of 5.6 to fully hydrate the phospholipid film, mix the solution evenly, and heat it at 50° C. for 60 minutes with ultrasonic treatment;
[0095] (3) Spray-dry the solution obtained in the above step (2) to...
Embodiment 3
[0103] Example 3 Preparation of Atorvastatin Calcium Liposome Particles
[0104] Shell (1000 bags):
[0105] Atorvastatin calcium liposome (calculated as atorvastatin calcium) 50g
[0106] Starch 150g
[0107] Sucrose 850g
[0108] Povidone K 30 10g
[0109] Orange flavor 30g
[0110] Preparation Process:
[0111] (1) pulverize the liposome containing 50g atorvastatin calcium, cross 80 mesh sieves, and set aside;
[0112] (2) take by weighing 150g starch, 850g sucrose cross 80 mesh sieves and mix, set aside;
[0113] (3) Mix the above raw and auxiliary materials evenly, then mix evenly with 30g orange essence, add 3% povidone K 30 330ml of 80% ethanol solution is used to make soft materials, passed through a 20-mesh sieve to granulate, dried at 60°C, granulated through a 18-mesh sieve, and set aside;
[0114] (4) Divide the dried granules to prepare atorvastatin calcium liposome granules.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com