Drug sustained-release composition and preparation method
A sustained-release composition and drug technology, which is applied in drug combinations, pharmaceutical formulations, antineoplastic drugs, etc., can solve the problems of poor uniformity of microspheres and drug loading, low drug loading and encapsulation efficiency, and reduce economic burden , Stable physical and chemical properties, good stability effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
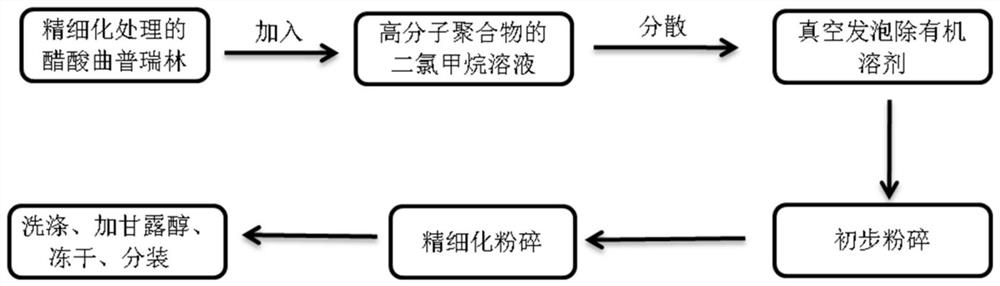
[0050] The second aspect of the present invention provides a preparation method of the drug sustained-release composition, sometimes referred to herein as "the preparation method of the present invention", which is a method using foaming and jet milling techniques. Specifically, the preparation method of the present invention at least includes the following steps:
[0051] (1) disperse triptorelin acetate dry powder in the solution of fat-soluble polymer to obtain dispersion liquid, wherein the concentration of solvent in the solution is 50% by weight-90% by weight;
[0052] (2) reducing the concentration of the solvent in the dispersion to 5% by weight to 25% by weight to obtain a concentrated solution;
[0053] (3) Foaming the concentrated solution to remove the solvent under negative pressure to obtain a foamed coagulation;
[0054] (4) Preliminary crushing of the foamed coagulum to obtain a coarse powder, and then secondary crushing by jet milling to form a fine powder; a...
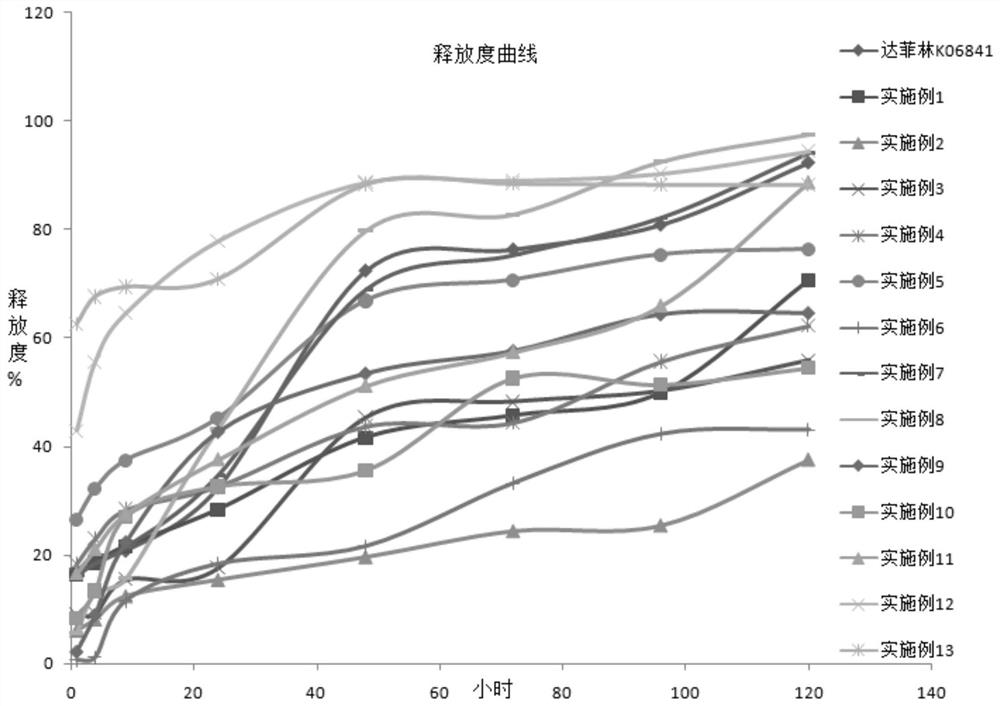
Embodiment 1
[0068] Weigh 50 mg (1.5%) of triptorelin acetate into a 30 ml vial, add 2 ml of purified water to fully dissolve, rapidly cool and freeze in liquid nitrogen, and freeze dry in a lyophilizer. Add high molecular polymer (PLGA / PLA) 2g (65.5%) in vial (high molecular polymer (PLGA / PLA) model sees Table 1, and different models represent the mass ratio of different lactic acid and glycolic acid, the same below The greater the number followed by the letter under the ratio, the greater the molecular weight and the greater the viscosity of the polymer), 5ml of dichloromethane, sealed with a sealing plug, and mixed at a high speed for 20 minutes on the suspension instrument at a speed of 2500rpm, cooled for 30 minutes, and repeated mixing for 3 times Shake overnight at 25°C.
[0069] Use an ultrasonic cell pulverizer to pulverize the suspension, with a power of 200 watts, ultrasonic for 20s, intermittent for 20s, and work for 10 minutes. Use an ice-water mixture to cool down the bottle ...
Embodiment 2-13
[0071] The pharmaceutical composition was prepared in a similar manner to Example 1 except that the composition of each component was changed as shown in Table 1.
[0072] Table 1 Compositions prepared by different types of high molecular polymers
[0073]
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com