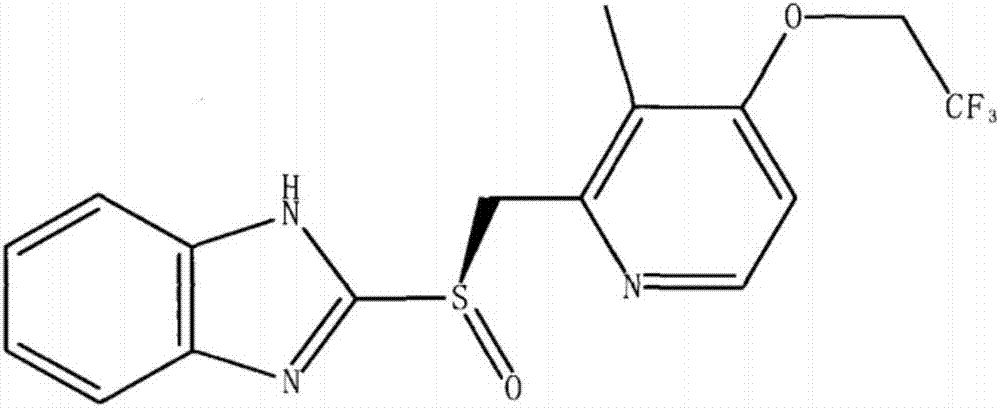
Dexlansoprazole crystal form and preparation method
A technology of dexlansoprazole crystals and crude dexlansoprazole, which is applied in the field of dexlansoprazole crystal forms and preparation, can solve problems such as product instability, and achieve long-term storage convenience and clinical drug safety , the effect of high drug safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] The preparation of embodiment 1 dexlansoprazole crystal form
[0025] In the 1000ml flask, add 10g dexlansoprazole crude product, 100ml acetone, stir and dissolve. Add 0.5 g of activated carbon, stir and decolorize for 0.5 hours, and filter. Control the temperature at 20° C., add 40 ml of isopropyl ether to the filtrate, stir and grow the crystals for 0.5 hours, tiny crystals appear, and the solution becomes turbid. Continue to dropwise add 300ml of isopropyl ether, and the dropwise addition is completed in 3 hours. It was filtered and vacuum-dried to obtain 8.99 g of white crystalline powder with an HPLC purity of 99.91%.
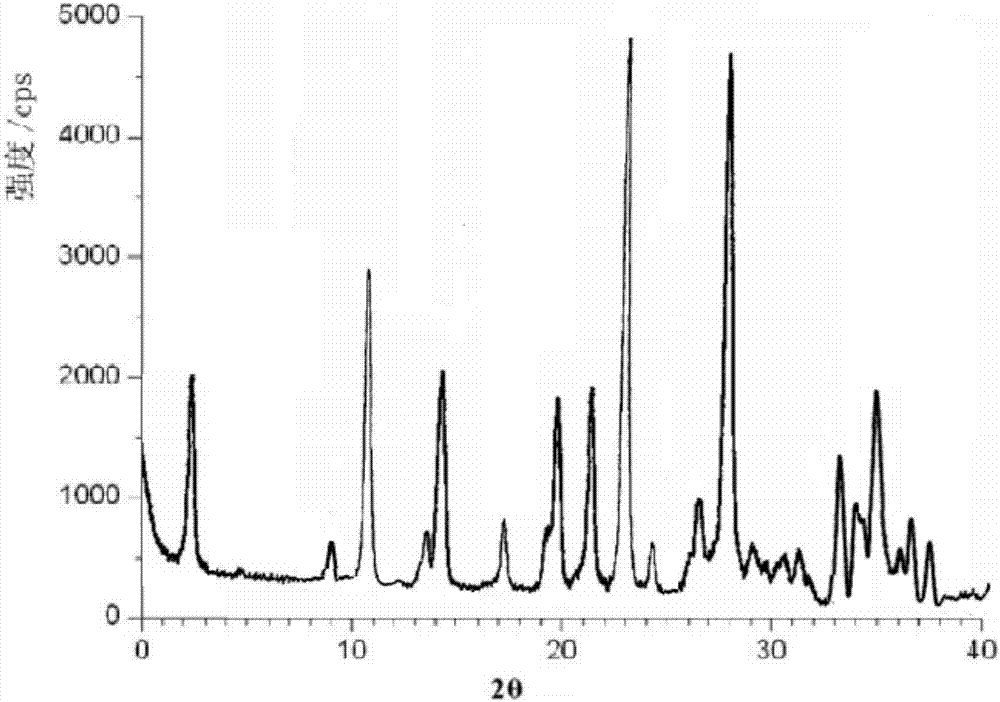
[0026] Prepared dexlansoprazole is measured by powder X-ray diffractometry method, obtains X-ray powder diffraction collection of patterns such as figure 1 shown.
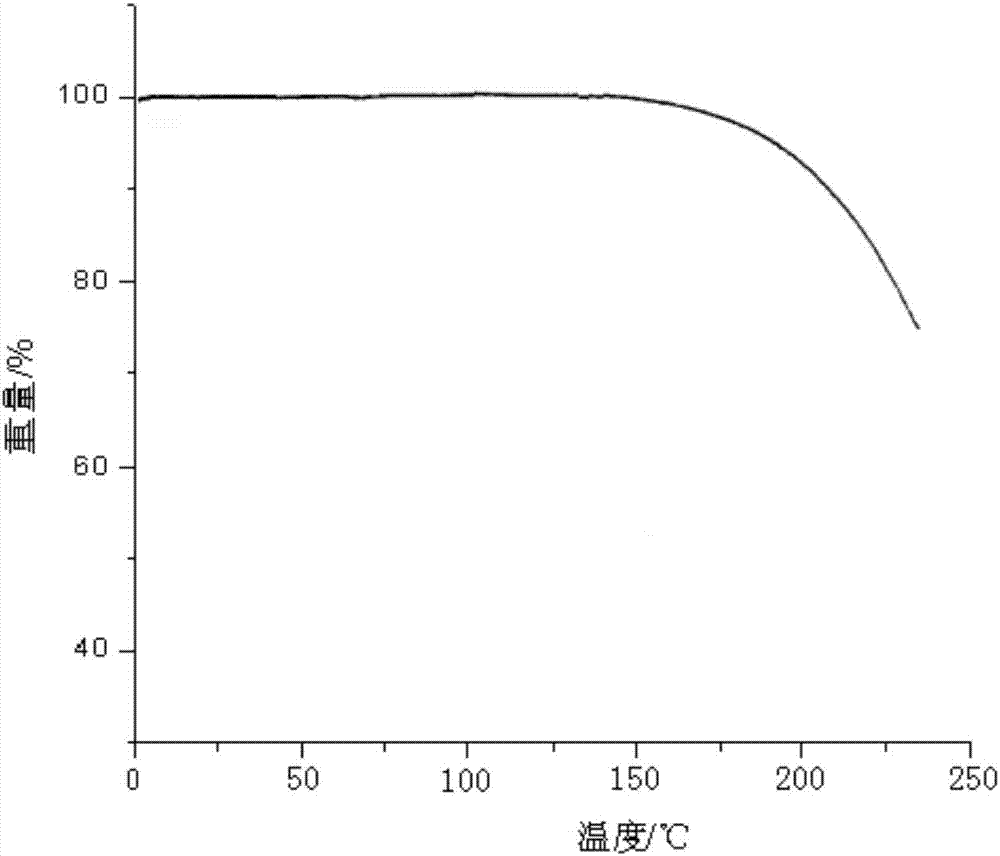
[0027] Using the PE Pyris Diamond TG thermogravimetric analyzer of Perkin-Elmer Company in the United States, the thermogravimetric analysis spectrum is as follows figure 2 As show...
Embodiment 2
[0028] The preparation of embodiment 2 dexlansoprazole crystal forms
[0029] In the 1000ml flask, add 10g dexlansoprazole crude product, 100ml acetone, stir and dissolve. Add 0.5 g of activated carbon, stir and decolorize for 0.5 hours, and filter. Control the temperature at 20° C., add 60 ml of isopropyl ether to the filtrate, stir and grow the crystals for 1 hour, tiny crystals appear, and the solution becomes turbid. Continue to dropwise add 350ml of isopropyl ether, and the dropwise addition is completed in 3 hours. It was filtered and vacuum-dried to obtain 9.21 g of white crystalline powder with an HPLC purity of 99.97%.
[0030] According to the XPRD data, the obtained crystal form is consistent with the crystal form in Example 1. The thermogravimetric analysis spectrum obtained by using the PEPyris Diamond TG thermogravimetric analyzer of Perkin-Elmer Company of the United States is consistent with that of Example 1.
Embodiment 3
[0031] The preparation of embodiment 3 dexlansoprazole crystal forms
[0032] In the 1000ml flask, add 10g dexlansoprazole crude product, 100ml acetone, stir and dissolve. Add 0.5 g of activated carbon, stir and decolorize for 0.5 hours, and filter. Control the temperature at 20° C., add 70 ml of isopropyl ether to the filtrate, stir and grow the crystals for 0.5 hours, tiny crystals appear, and the solution becomes turbid. Continue to dropwise add 300ml of isopropyl ether, and the dropwise addition is completed in 3 hours. It was filtered and vacuum-dried to obtain 9.08 g of white crystalline powder with an HPLC purity of 99.93%.
[0033] According to the XPRD data, the obtained crystal form is consistent with the crystal form in Example 1. The thermogravimetric analysis spectrum obtained by using the PEPyris Diamond TG thermogravimetric analyzer of Perkin-Elmer Company of the United States is consistent with that of Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com