Refining method of 2,6-diamino-3,5-dinitropyridine-1-oxide
The technology of a dinitropyridine and a refining method is applied in the refining field of 2,6-diamino-3,5-dinitropyridine-1-oxide, and can solve the problem of large consumption of organic solvent and solvent trifluoroacetic acid. Problems such as high toxicity, to achieve the effect of low solvent toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
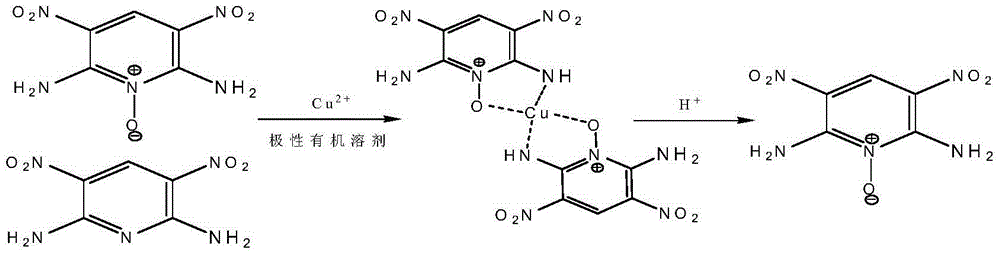
[0028] (1)Cu(ANPyO) 2 Preparation of complexes
[0029] In a 100ml three-necked flask equipped with a magnet and a thermometer, add 43.0mLDMSO and 4.30g of 2,6-diamino-3,5-dinitropyridine-1 oxide (20mmol) with a purity of 90% under stirring , after adding, heat to 80°C, keep warm for 0.5h, then add 2.42g Cu(NO 3 ) 2 ·3H 2 O (10mmol), keep warm for 2h, filter while hot, recover the DMSO filtrate, wash the filter cake with water, wash with acetone, and dry to obtain 4.40g of 2,6-diamino-3,5-dinitropyridine-1-oxide Copper complex, yield 99.0%.
[0030] Structure Identification:
[0031] DSC decomposition peak temperature: 384.1°C;
[0032] Infrared spectrum (KBr, cm -1 )γ: 3405, 3356, 3287, 3080, 1635, 604, 546;
[0033] The above structural identification data confirm that the substance obtained in this step is 2,6-diamino-3,5-dinitropyridine-1-oxide copper complex.
[0034] (2) Acidification to remove copper ions to obtain ANPyO
[0035] In a 100ml three-necked flask eq...
Embodiment 2
[0042] (1)Cu(ANPyO) 2 Preparation of complexes
[0043] In a 100ml three-necked flask equipped with a magnet and a thermometer, 86.0mL DMF and 4.30g of 2,6-diamino-3,5-dinitropyridine-1 oxide (20mmol ), heated to 80°C after the addition, kept the temperature for 0.5h, then added 2.40g Cu(OOCCH 3 ) 2 ·H 2 O (12mmol), keep warm for 4h, filter while hot, recover the DMF filtrate, wash the filter cake with water, wash with acetone, and dry to obtain 4.50g of 2,6-diamino-3,5-dinitropyridine-1-oxide Copper complex, yield 97.9%.
[0044] (2) Acidification to remove copper ions to obtain ANPyO
[0045] In a 250ml three-neck flask equipped with a stirrer and a thermometer, add 17.3ml of sulfuric acid, and add 2.47g (5mmol) of the above-mentioned 2,6-diamino-3,5-dinitropyridine-1 oxide copper complex under stirring After the addition, the temperature was raised to 80°C. After the solid matter was completely dissolved, it was kept warm for 0.5h, and the insoluble copper sulfate sol...
Embodiment 3
[0047] (1)Cu(ANPyO) 2 Preparation of complexes
[0048] In a 100ml three-necked flask equipped with a magnet and a thermometer, add 50mL DMSO and 4.30g of 2,6-diamino-3,5-dinitropyridine-1 oxide (20mmol) with a purity of 93% under stirring , after adding, heat to 80°C, keep warm for 0.5h, then add 3.00g Cu(SO 4 ) 2 ·5H 2 O (12mmol), keep warm for 1h, filter while hot, recover the DMSO filtrate, wash the filter cake with water, wash with acetone, and dry to obtain 4.40g of 2,6-diamino-3,5-dinitropyridine-1-oxide Copper complex, yield 95.8%.
[0049] (2) Acidification to remove copper ions to obtain ANPyO
[0050] In a 100ml three-necked flask equipped with a stirrer and a thermometer, add 12ml of sulfuric acid, and add 2.47g (5mmol) of the above-mentioned 2,6-diamino-3,5-dinitropyridine-1 oxide copper complex under stirring, After the addition, the temperature was raised to 80°C. After the solid matter was completely dissolved, the heat was kept for 0.5h. The insoluble co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com