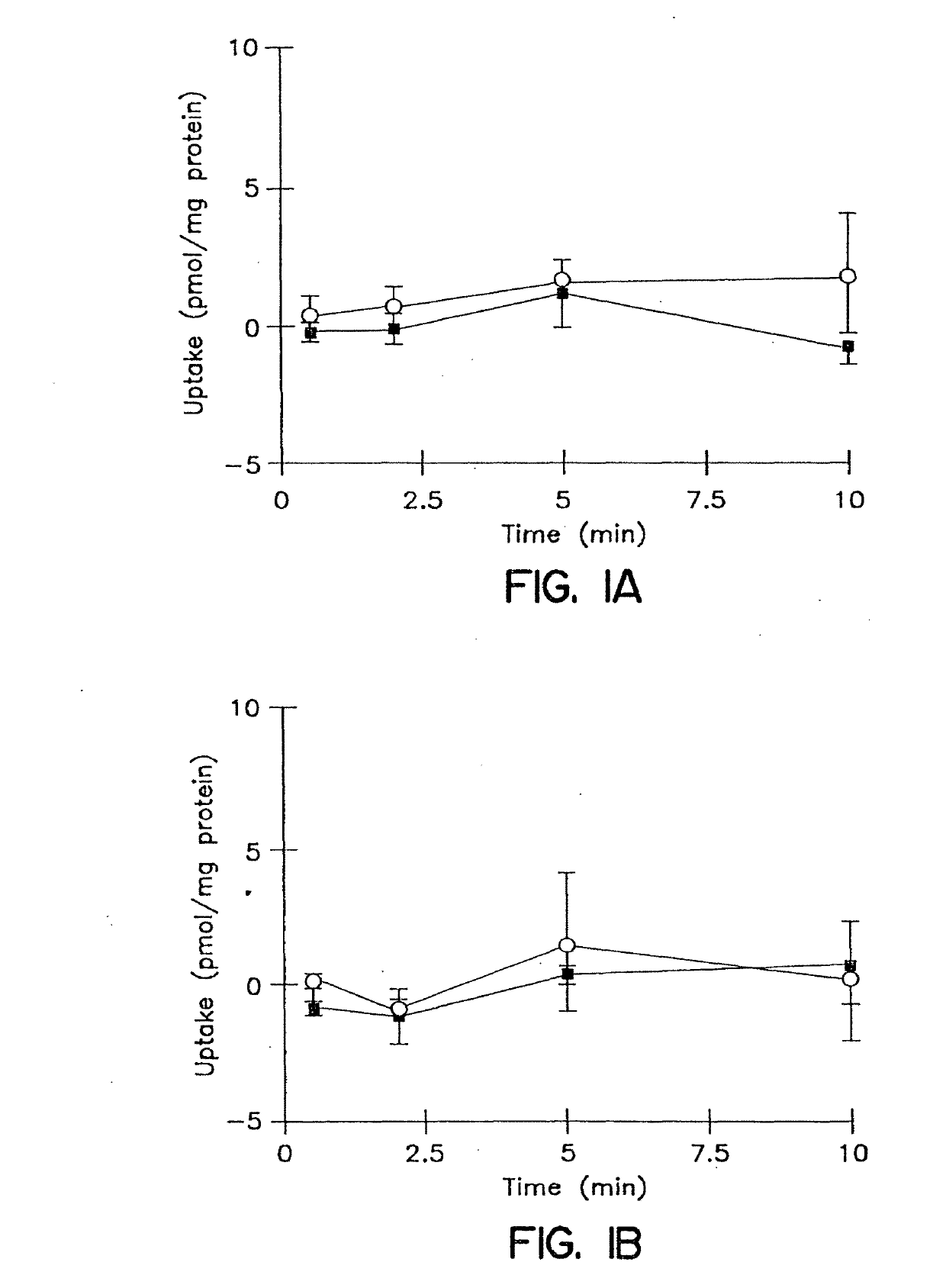
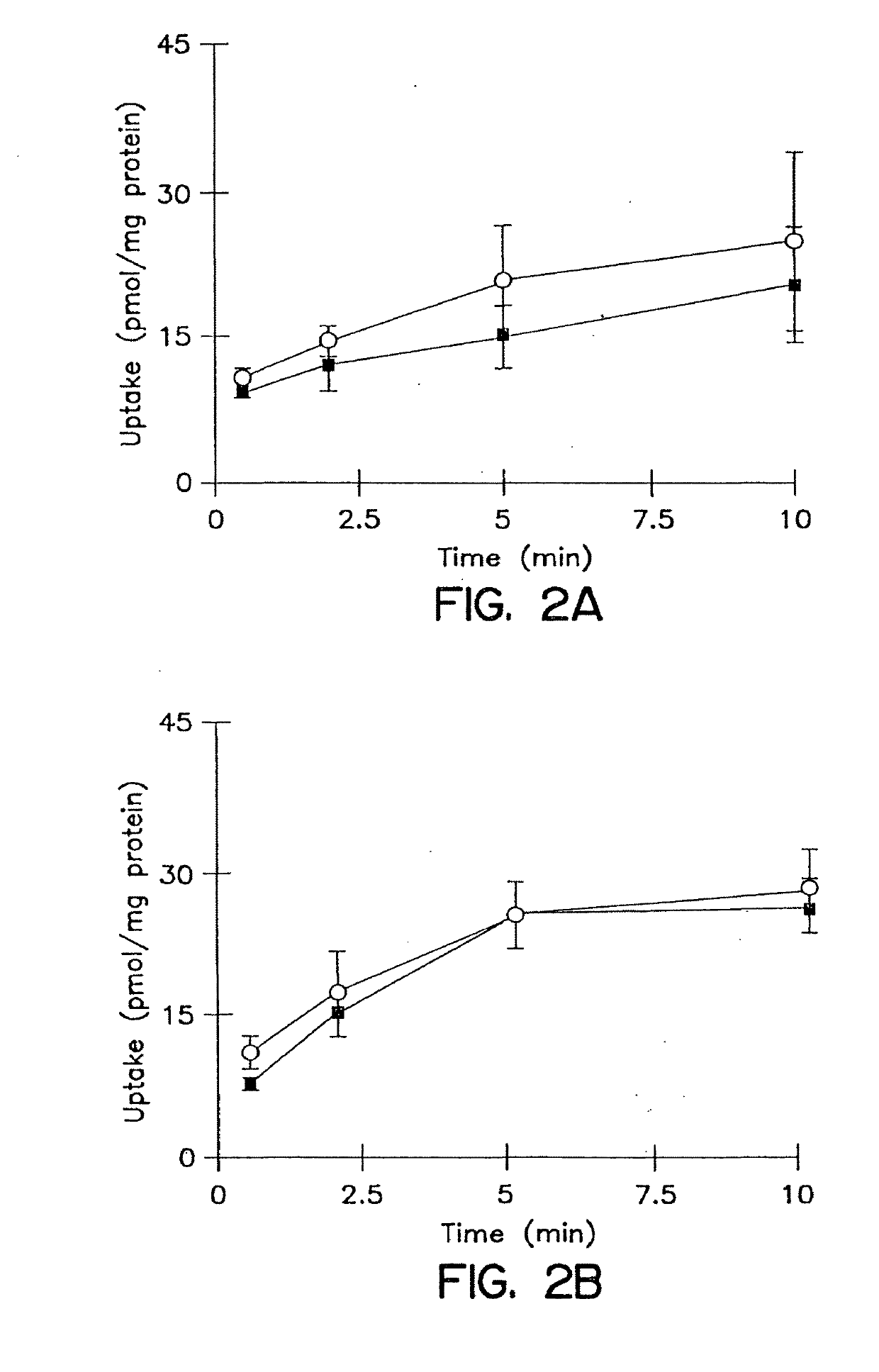
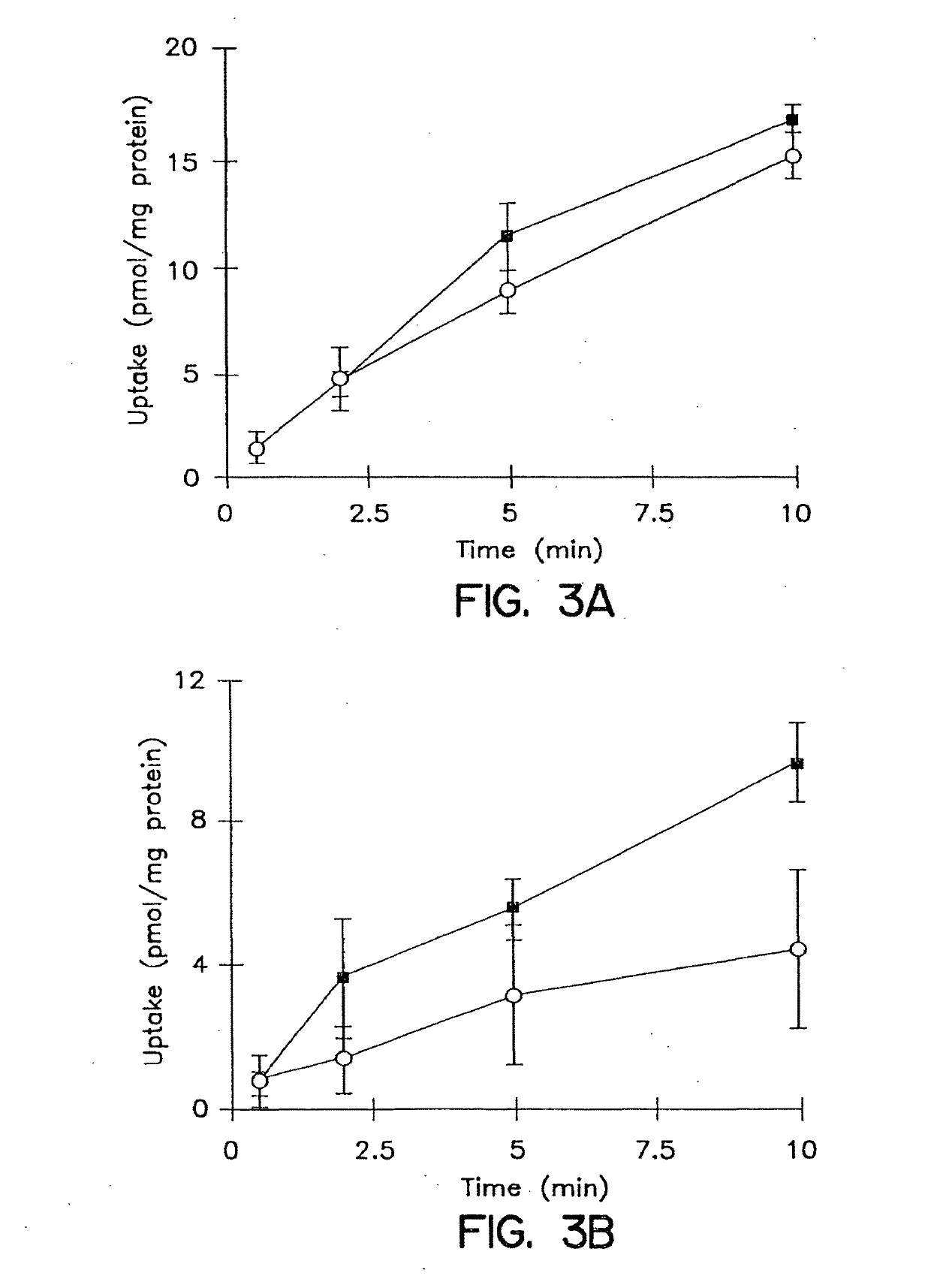
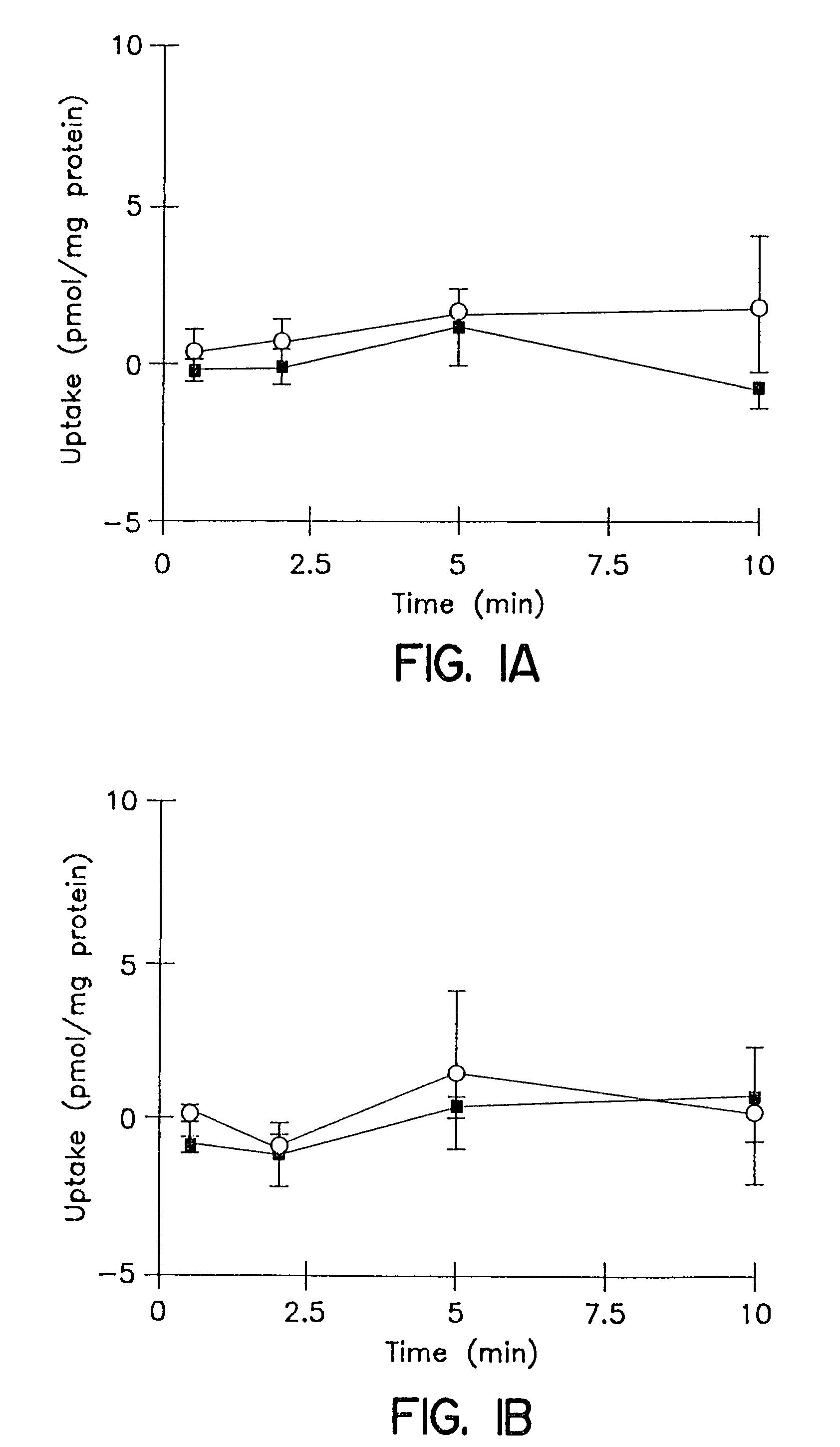
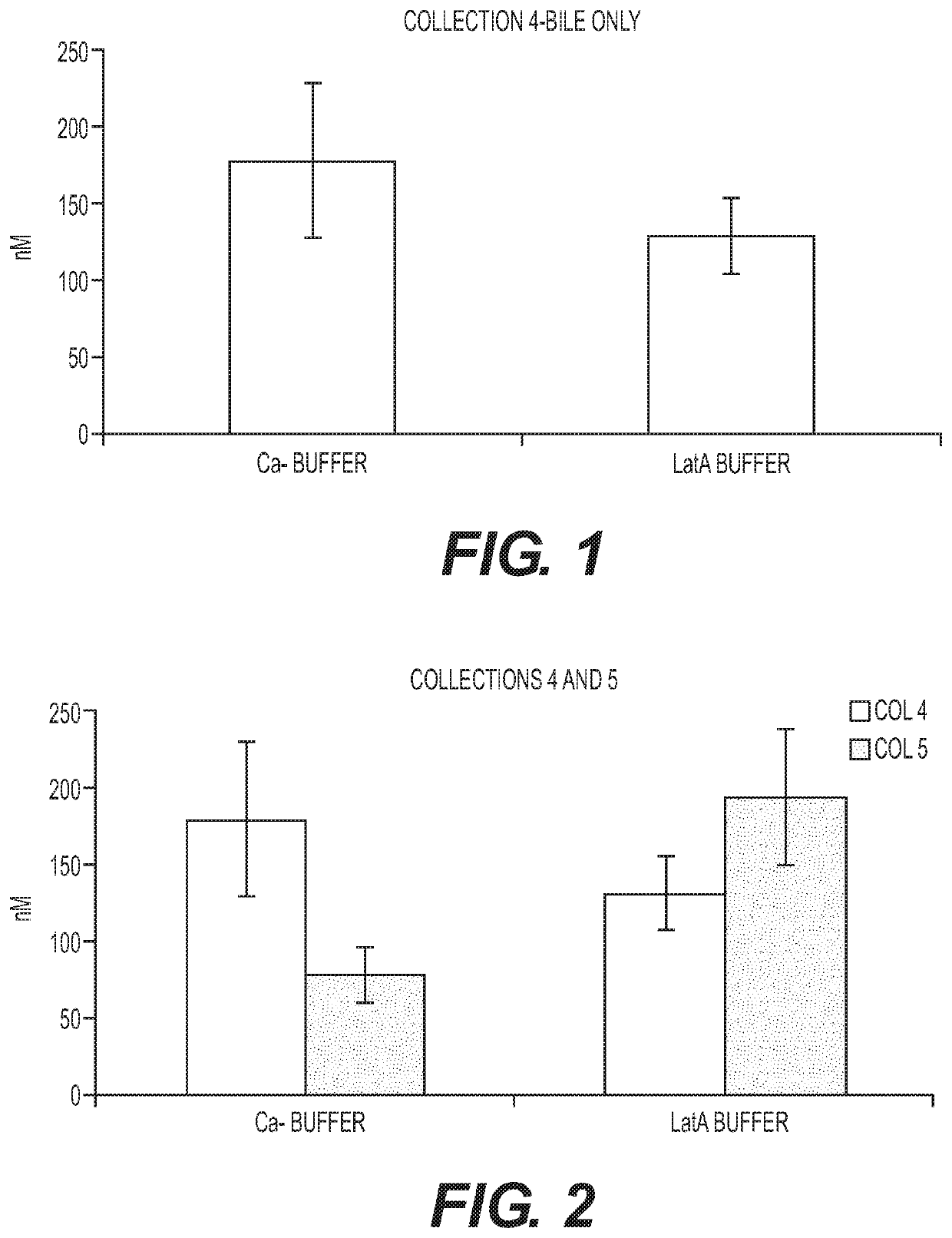
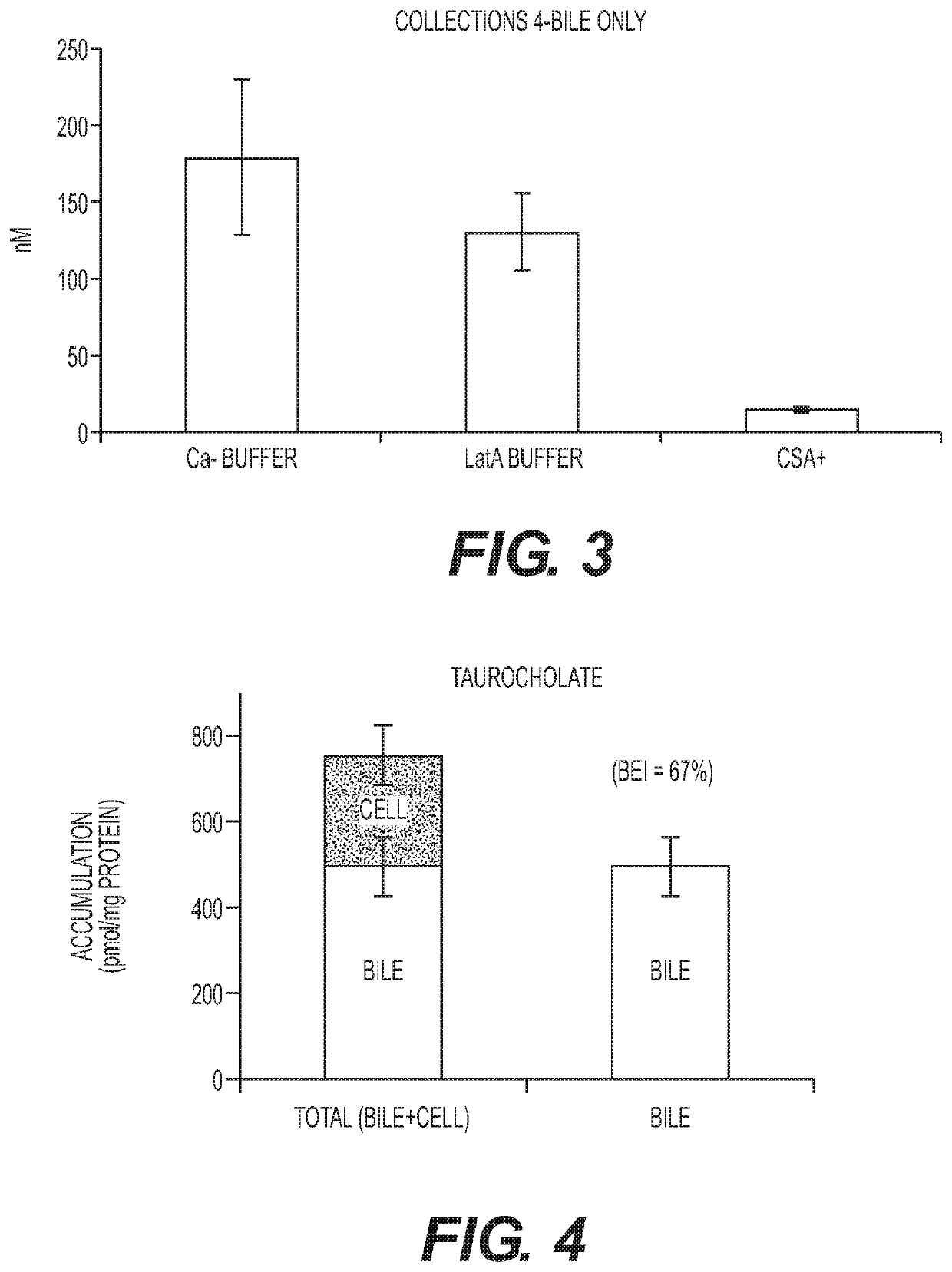
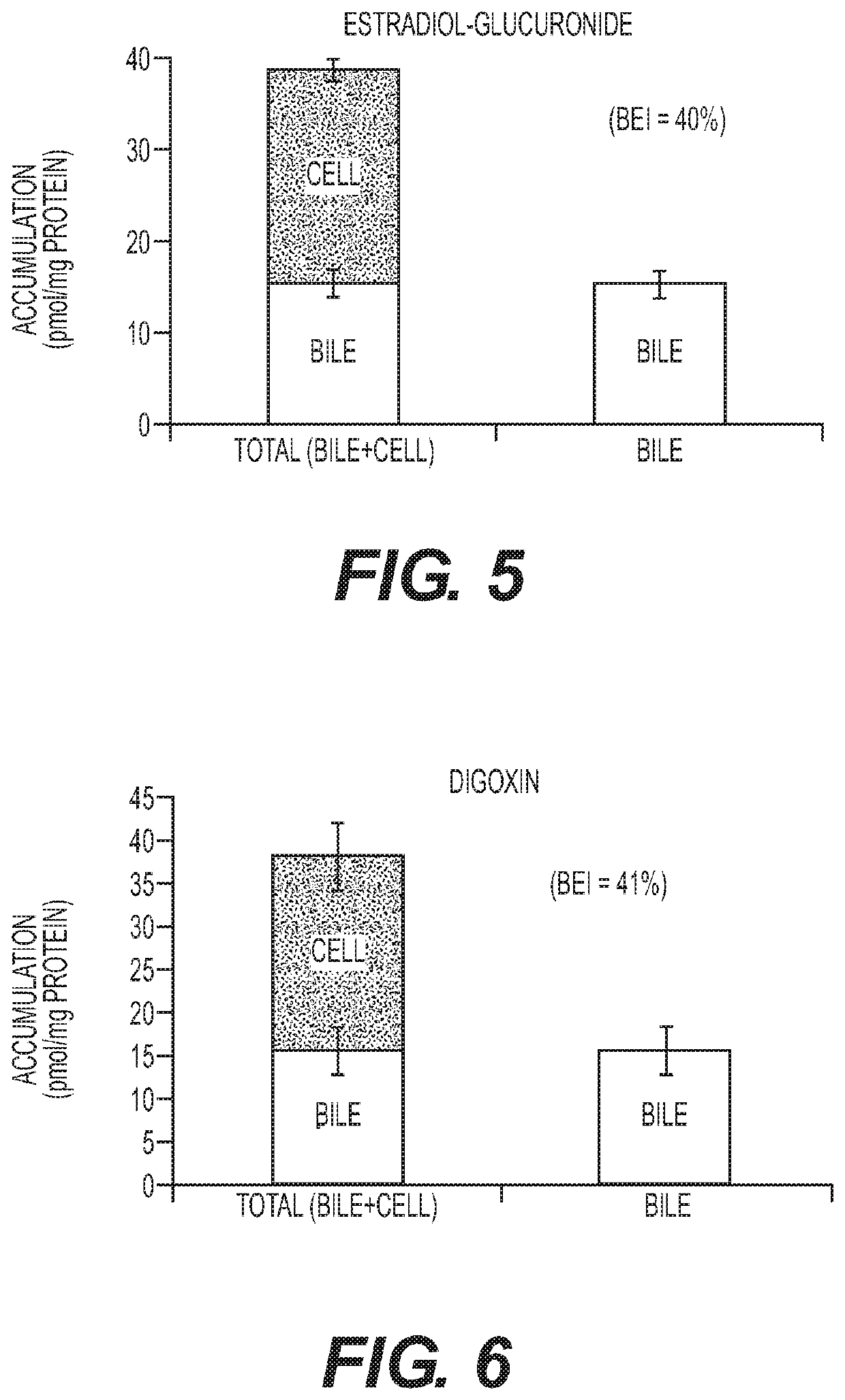
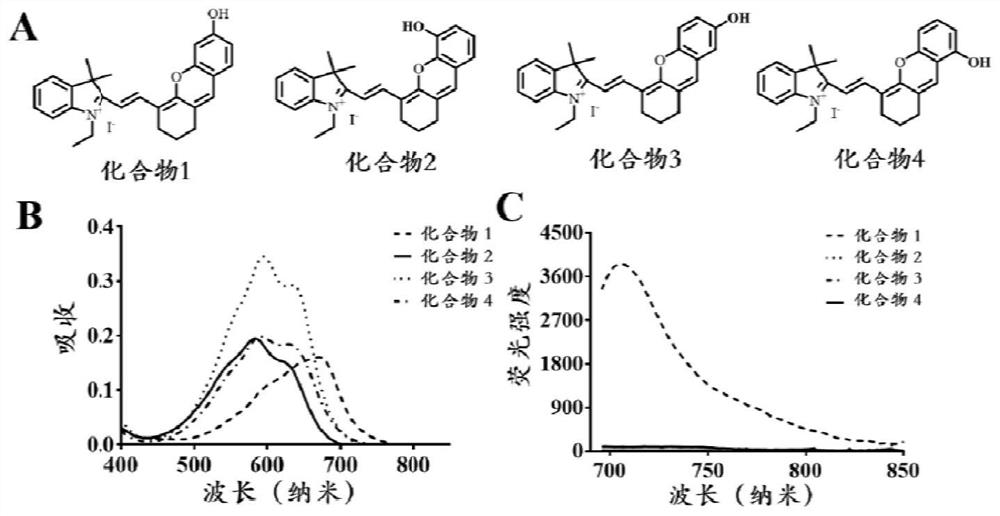
Patents
Literature
34 results about "Biliary excretion" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
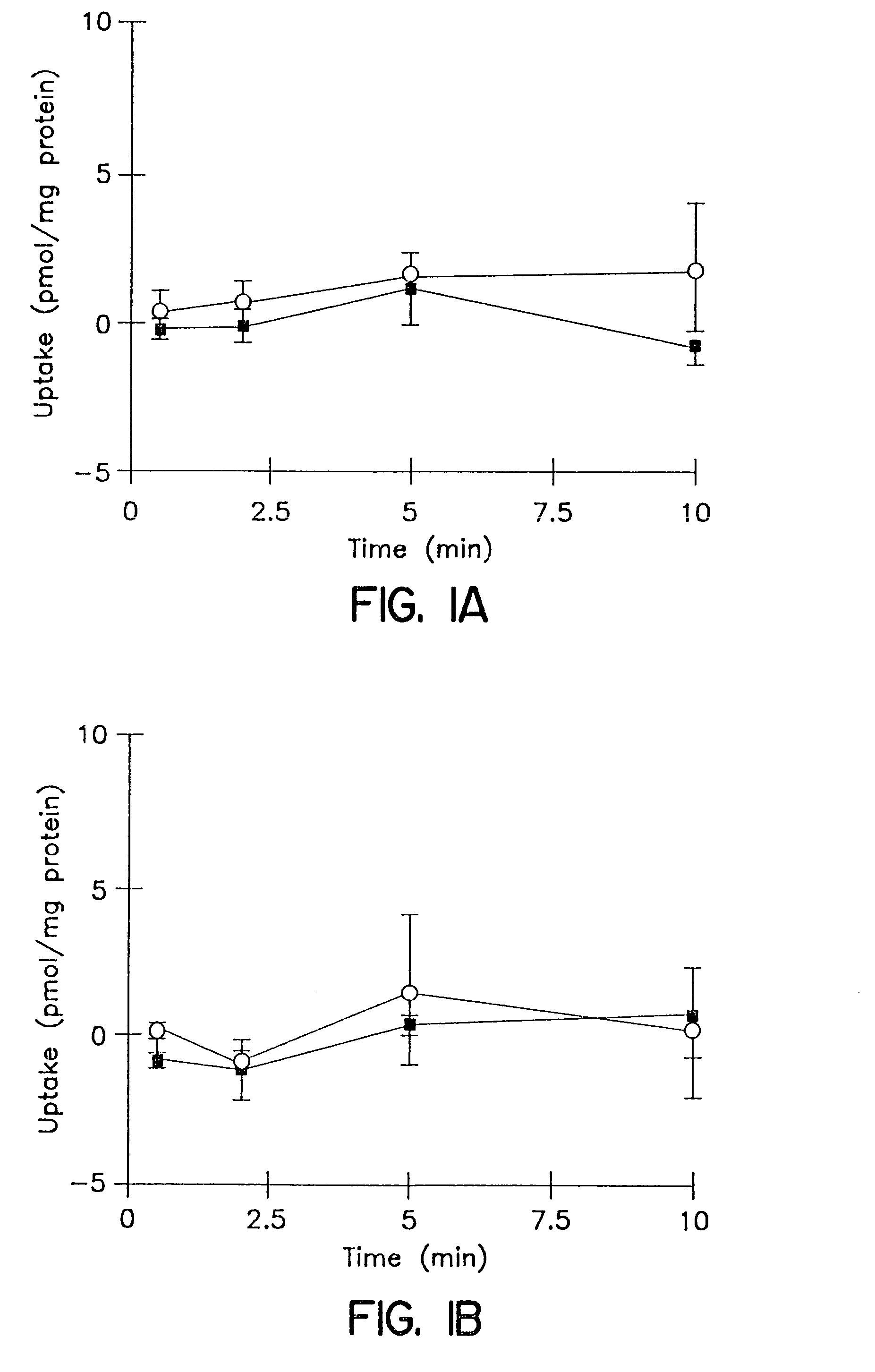
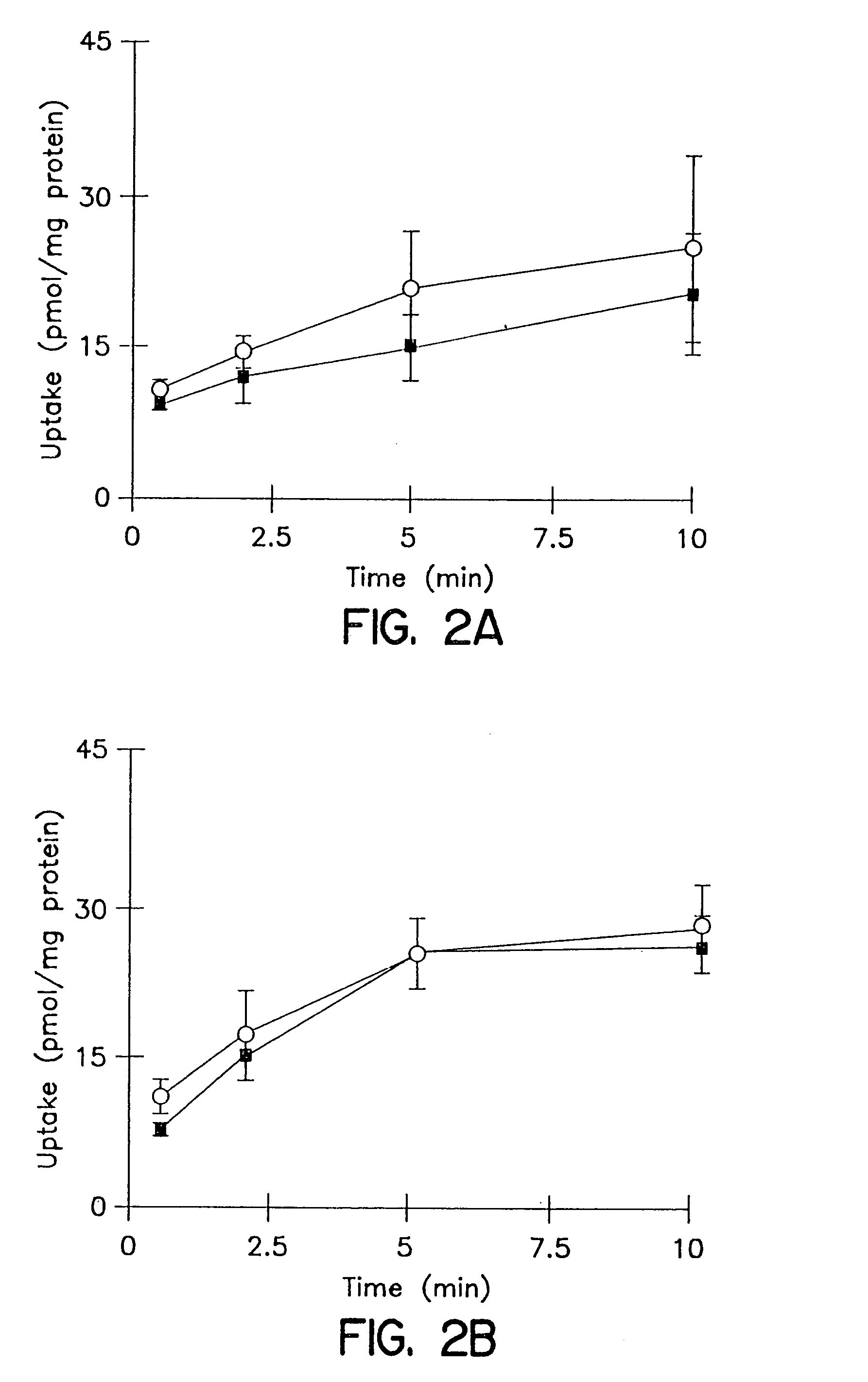
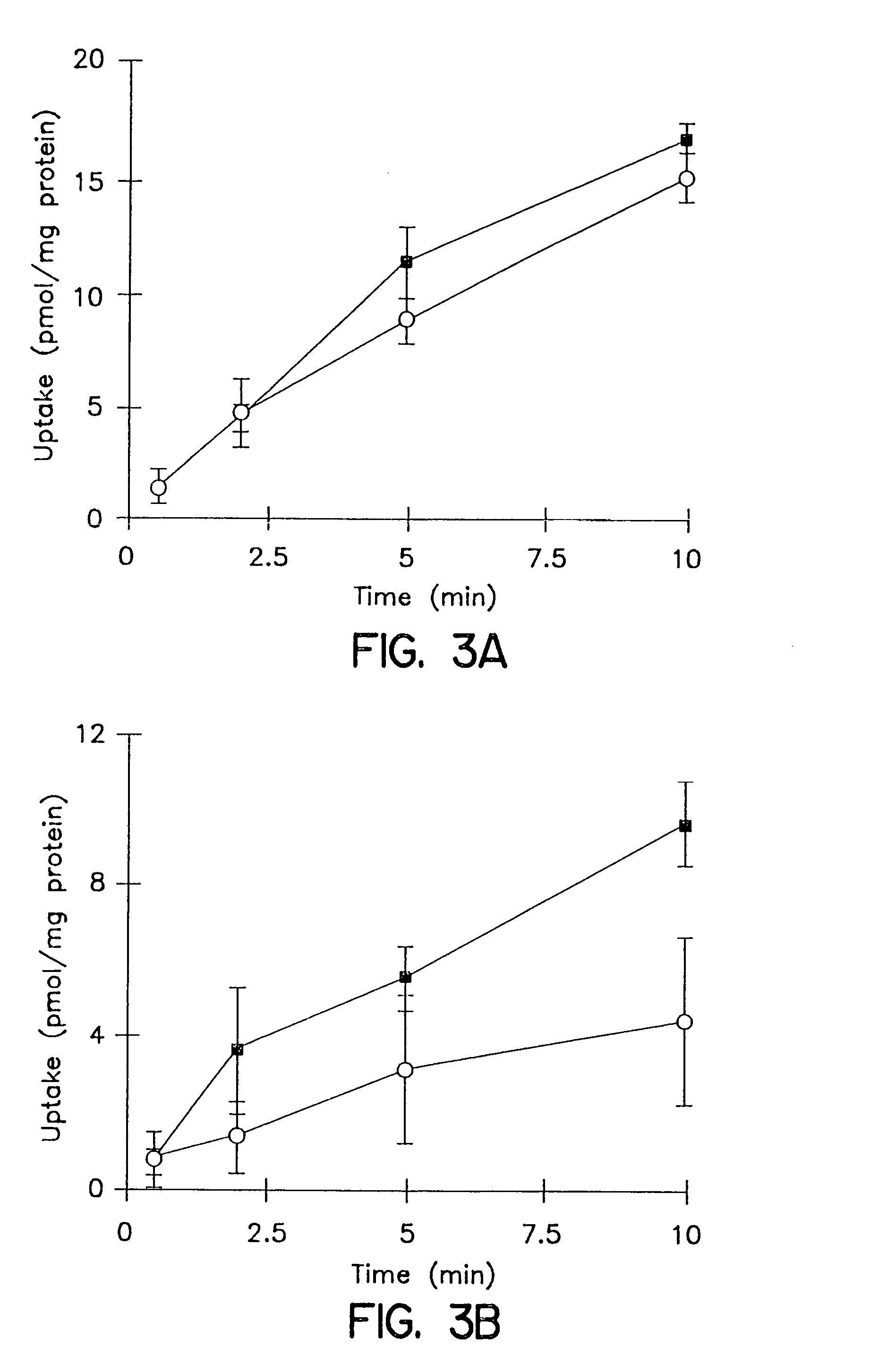
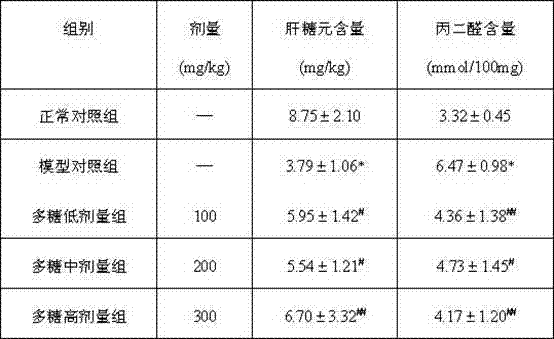
Biliary Excretion. Biliary excretion involves active secretion of drug molecules or their metabolites from hepatocytes into the bile. The bile then transports the drugs to the gut, where the drugs are excreted. The transport process is similar to those described for renal tubular secretion.
Medical devices having a temporary radiopaque coating
A medical device comprising radiopaque water-dispersible metallic nanoparticles, wherein the nanoparticles are released from the medical device upon implantation of the device. The medical device of the present invention is sufficiently radiopaque for x-ray visualization during implantation, but loses its radiopacity after implantation to allow for subsequent visualization using more sensitive imaging modalities such as CT or MRI.The nanoparticles are formed of a metallic material and have surface modifications that impart water-dispersibility to the nanoparticles. The nanoparticles may be any of the various types of radiopaque water-dispersible metallic nanoparticles that are known in the art. The nanoparticles may be adapted to facilitate clearance through renal filtration or biliary excretion. The nanoparticles may be adapted to reduce tissue accumulation and have reduced toxicity in the human body. The nanoparticles may be applied directly onto the medical device, e.g., as a coating, or be carried on the surface of or within a carrier coating on the medical device, or be dispersed within the pores of a porous layer or porous surface on the medical device. The medical device itself may be biodegradable and may have the nanoparticles embedded within the medical device itself or applied as or within a coating on the biodegradable medical device. The nanoparticles may be released by diffusion through the carrier coating, disruption of hydrogen bonds between the nanoparticles and the carrier coating, degradation of the nanoparticle coating, degradation of the carrier coating, diffusion of the nanoparticles from the medical device, or degradation of the medical device carrying the nanoparticles.
Owner:BOSTON SCI SCIMED INC
Method of screening candidate compounds for susceptibility to biliary excretion
InactiveUS7601494B2More compoundedFast processSugar derivativesGenetically modified cellsBiliary excretionHepatica
A method of screening a candidate compound for susceptibility to biliary excretion by a hepatocyte transport protein. The method includes the steps of providing a culture of hepatocytes comprising a transport protein, the culture having at least one bile canaliculus; exposing a candidate compound to the culture; and determining an amount of candidate compound in the at least one bile canaliculus, the amount of candidate compound in the at least one bile canaliculus indicating the susceptibility of the candidate compound to biliary excretion by the transport protein. In some embodiments determining the amount of candidate compound in the bile canaliculus comprises inhibiting expression of the transport protein, measuring the amount of candidate compound in the bile canaliculus and comparing amounts of compound in the canalicules with and without inhibition of the transport protein. A difference in the amount of candidate compound in the canaliculus indicates susceptibility of the candidate compound to biliary excretion by the transport protein. In one embodiment, expression of the transport protein is inhibited through introduction of a RNA having a sequence corresponding to a coding strand of the gene encoding the transport protein into the hepatocyte. Optionally, the culture of hepatocytes is a long-term culture in a sandwich configuration. The method is particularly applicable to the screening of multiple candidate compounds in a single effort.
Owner:THE UNIV OF NORTH CAROLINA AT CHAPEL HILL
Method for inhibiting expression of a protein in a hepatocyte
InactiveUS20090325297A1Fast processQuick filterSugar derivativesGenetically modified cellsBiliary excretionBile canaliculus
A method of screening a candidate compound for susceptibility to biliary excretion by a hepatocyte transport protein. The method includes the steps of providing a culture of hepatocytes comprising a transport protein, the culture having at least one bile canaliculus; exposing a candidate compound to the culture; and determining an amount of candidate compound in the at least one bile canaliculus, the amount of candidate compound in the at least one bile canaliculus indicating the susceptibility of the candidate compound to biliary excretion by the transport protein. In some embodiments determining the amount of candidate compound in the bile canaliculus comprises inhibiting expression of the transport protein, measuring the amount of candidate compound in the bile canaliculus and comparing amounts of compound in the canalicules with and without inhibition of the transport protein. A difference in the amount of candidate compound in the canaliculus indicates susceptibility of the candidate compound to biliary excretion by the transport protein. In one embodiment, expression of the transport protein is inhibited through introduction of a RNA having a sequence corresponding to a coding strand of the gene encoding the transport protein into the hepatocyte. Optionally, the culture of hepatocytes is a long-term culture in a sandwich configuration. The method is particularly applicable to the screening of multiple candidate compounds in a single effort.
Owner:THE UNIV OF NORTH CAROLINA AT CHAPEL HILL
Method of screening candidate compounds for susceptibility to biliary excretion by endogenous transport systems
InactiveUS7604934B2Inexpensive and rapid methodMore compoundedMicrobiological testing/measurementArtificial cell constructsTransport systemBiliary excretion
A method of screening a candidate compound for susceptibility to biliary excretion. The method includes the steps of providing a culture of hepatocytes, the culture having at least one bile canaliculus; exposing a candidate compound to the culture; and determining an amount of candidate compound in the at least one bile canaliculus, the amount of candidate compound in the at least one bile canaliculus indicating the susceptibility of the candidate compound to biliary excretion. Optionally, the culture of hepatocytes is a long-term culture in a sandwich configuration. The method is particularly applicable to the screening of multiple candidate compounds in a single effort.
Owner:THE UNIV OF NORTH CAROLINA AT CHAPEL HILL
Chinese medicinal herb pills for treating cholecystitis and gallstone
InactiveCN102526616AAlleviate inflammatory lesionsEasy to shrinkDigestive systemPill deliveryMedicinal herbsBile Juice
The invention relates to Chinese medicinal herb pills for treating cholecystitis and gallstone. The Chinese medicinal herb pills are prepared from 22 gouts of Chinese medicinal herbs, namely coptis root, baikal skullcap root, phellodendron, corydalis tuber, curcuma, costus root, loosestrife herb, Japanese climbing fern spore, dark plum, white peony root, fine-leaf schizonepeta herb, green tangerine peel, immature bitter orange, turmeric, polygonum cuspidatum, Szechwan chinaberry fruit, gentian, bupleurum, oriental wormwood, chicken's gizzard-membrane, compound of glauber-salt and liquorice and slaked lime. The pills can lighten the pathological change of cholecystitis, promote gallbladder contraction and promote gallstone excretion and biliary excretion; by the pills, biliary excretion components are radically changed, the metabolism requirement of the human body is met, calculi are gradually disintegrated and deposited, the micro environment in the gall bladder is recovered when the calculi are discharged, and the aim of thoroughly discharging the calculi is fulfilled. The Chinese medicinal herb pills have the advantages of quick response, short treatment course, high healing rate, low recurrence rate, treatment for both symptoms and root causes, no toxic or side effects and the like.
Owner:马金桥
Method for culturing animal hepatocyte
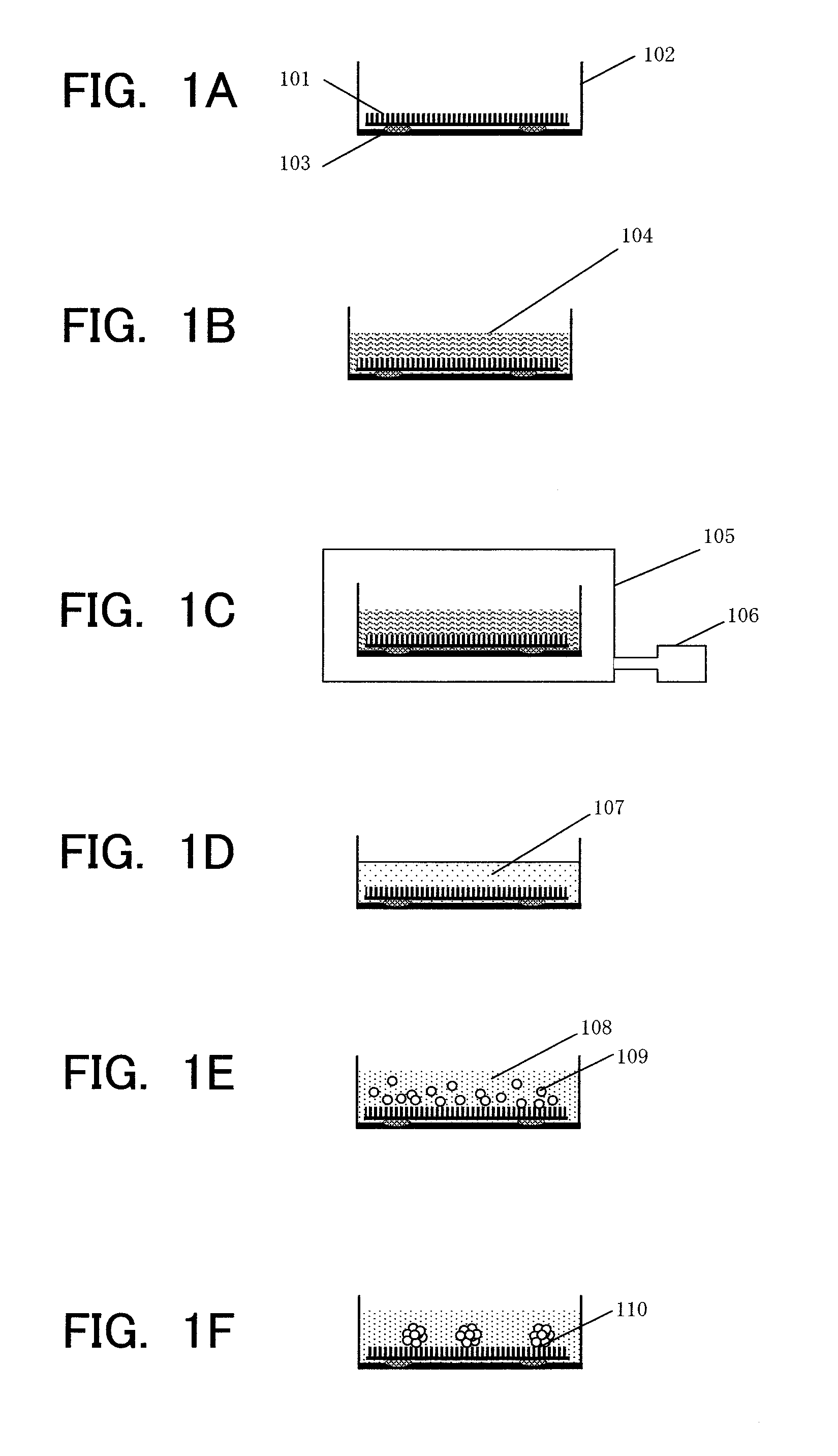
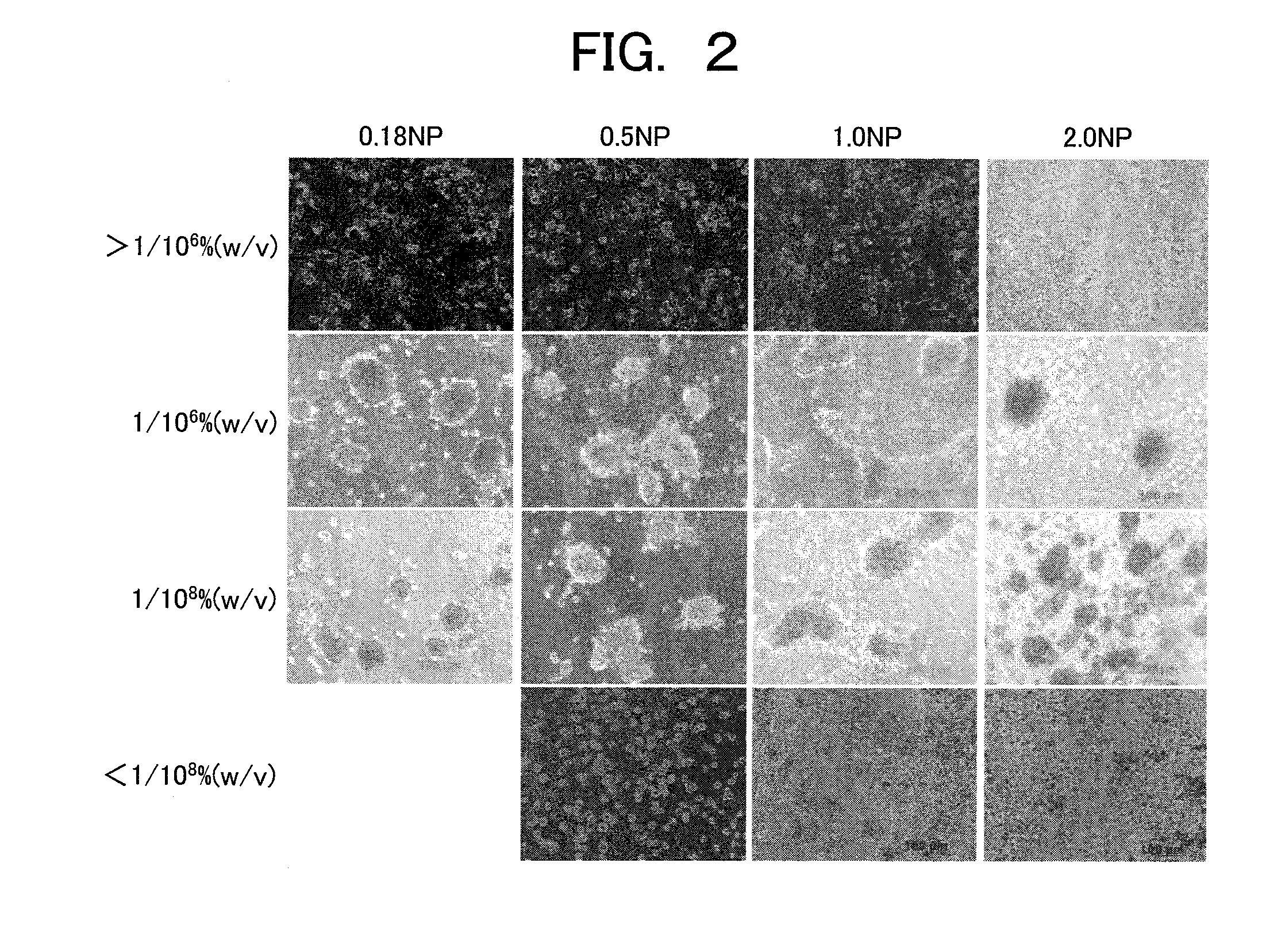
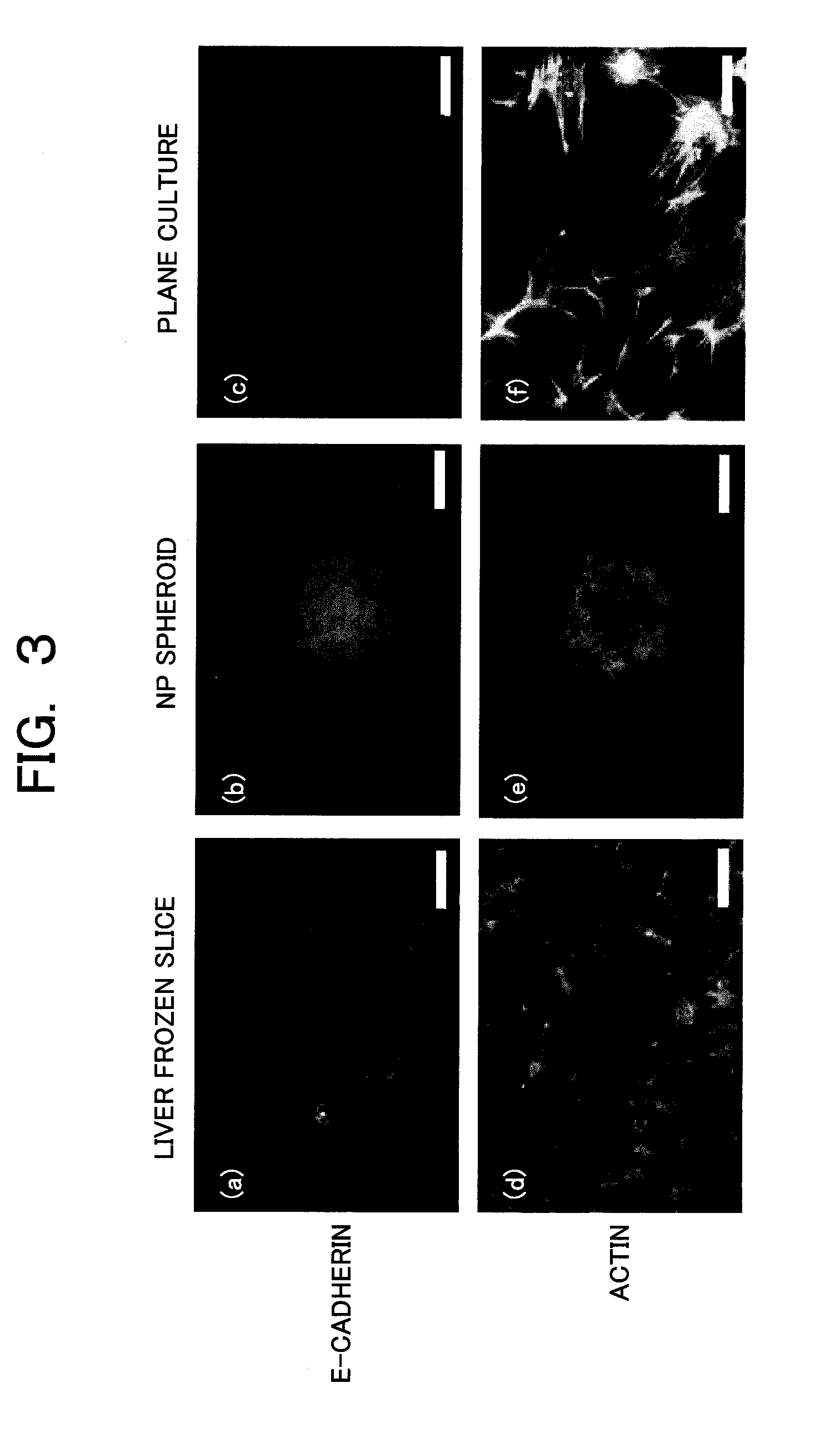
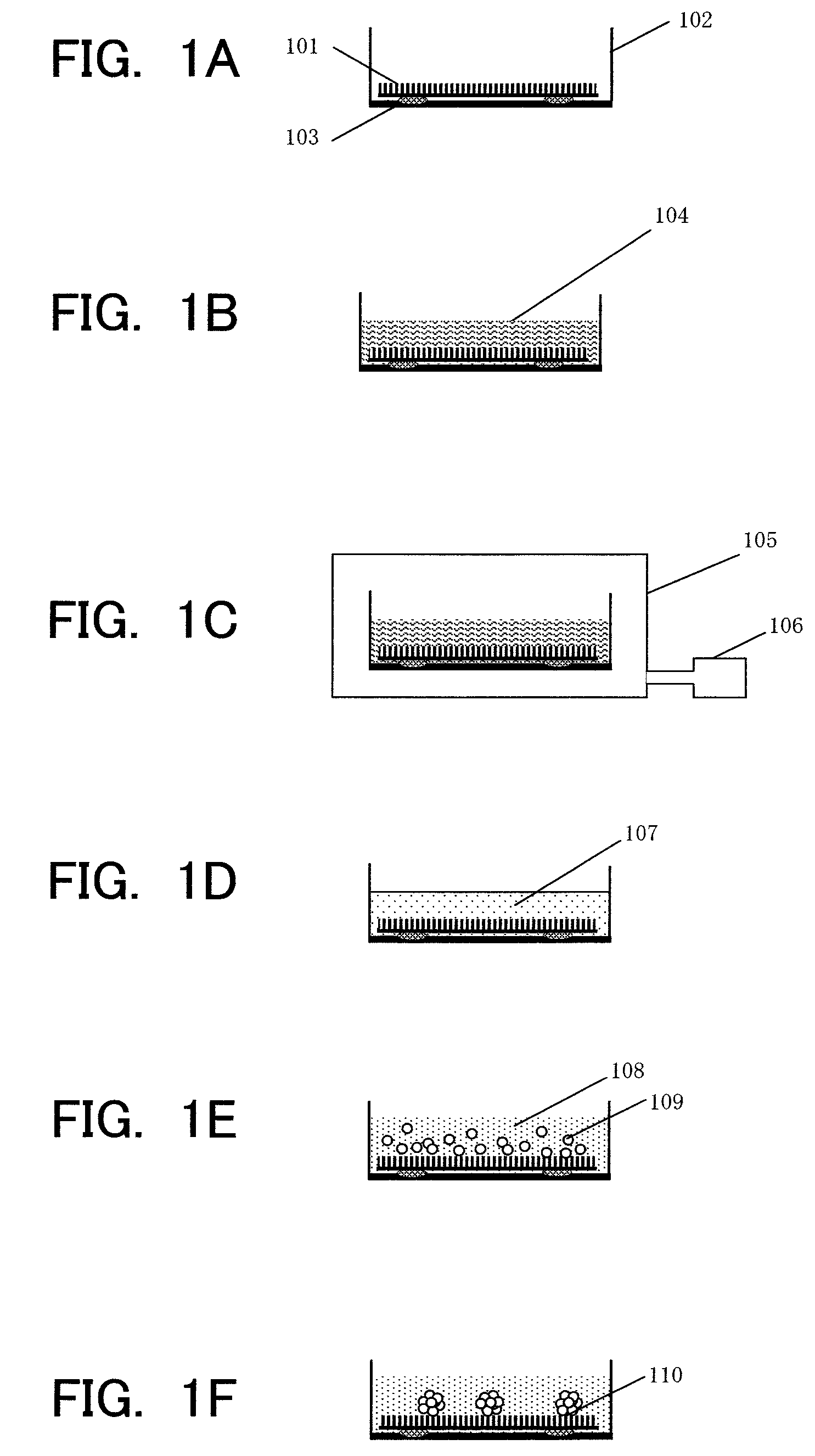
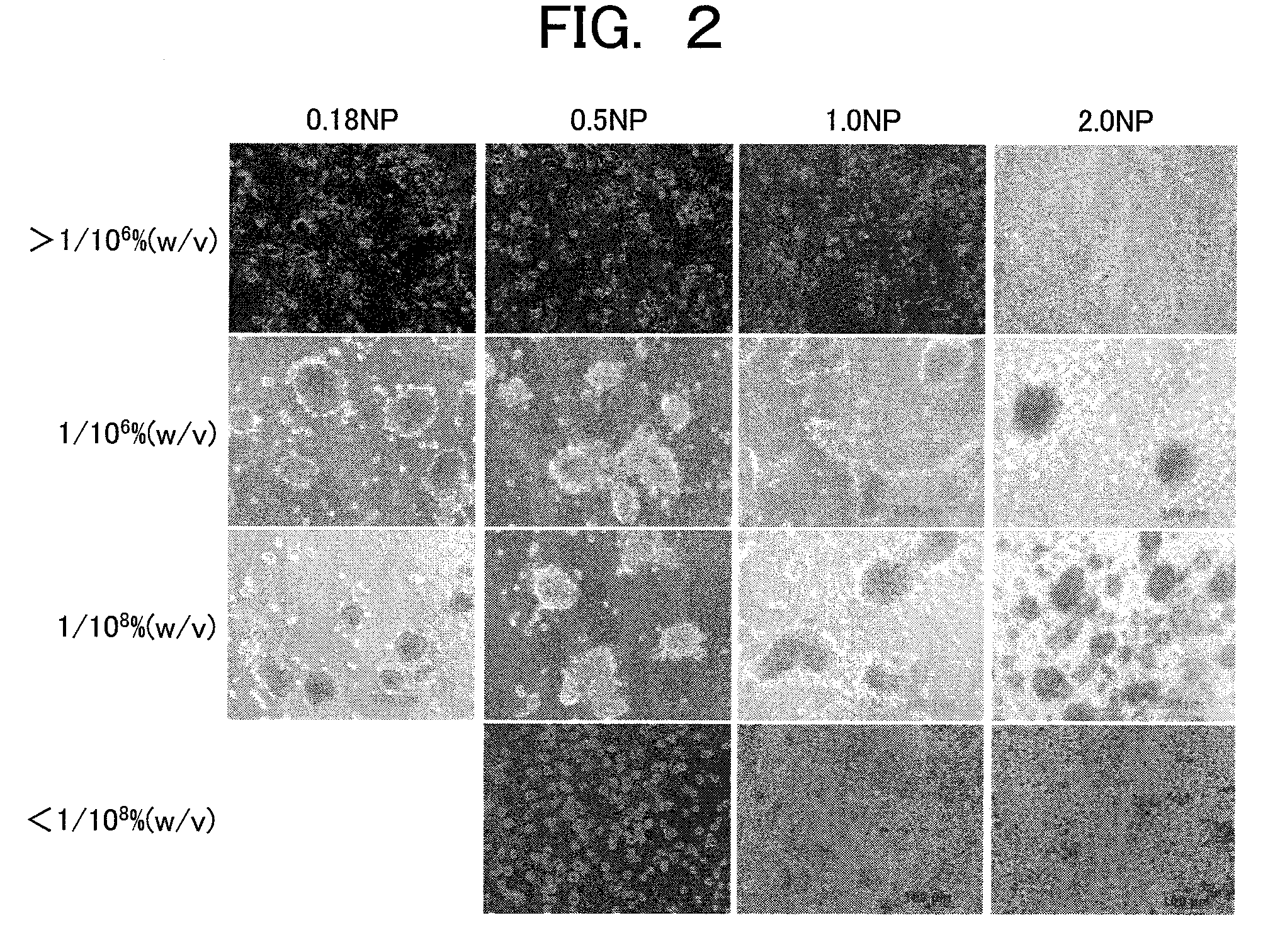
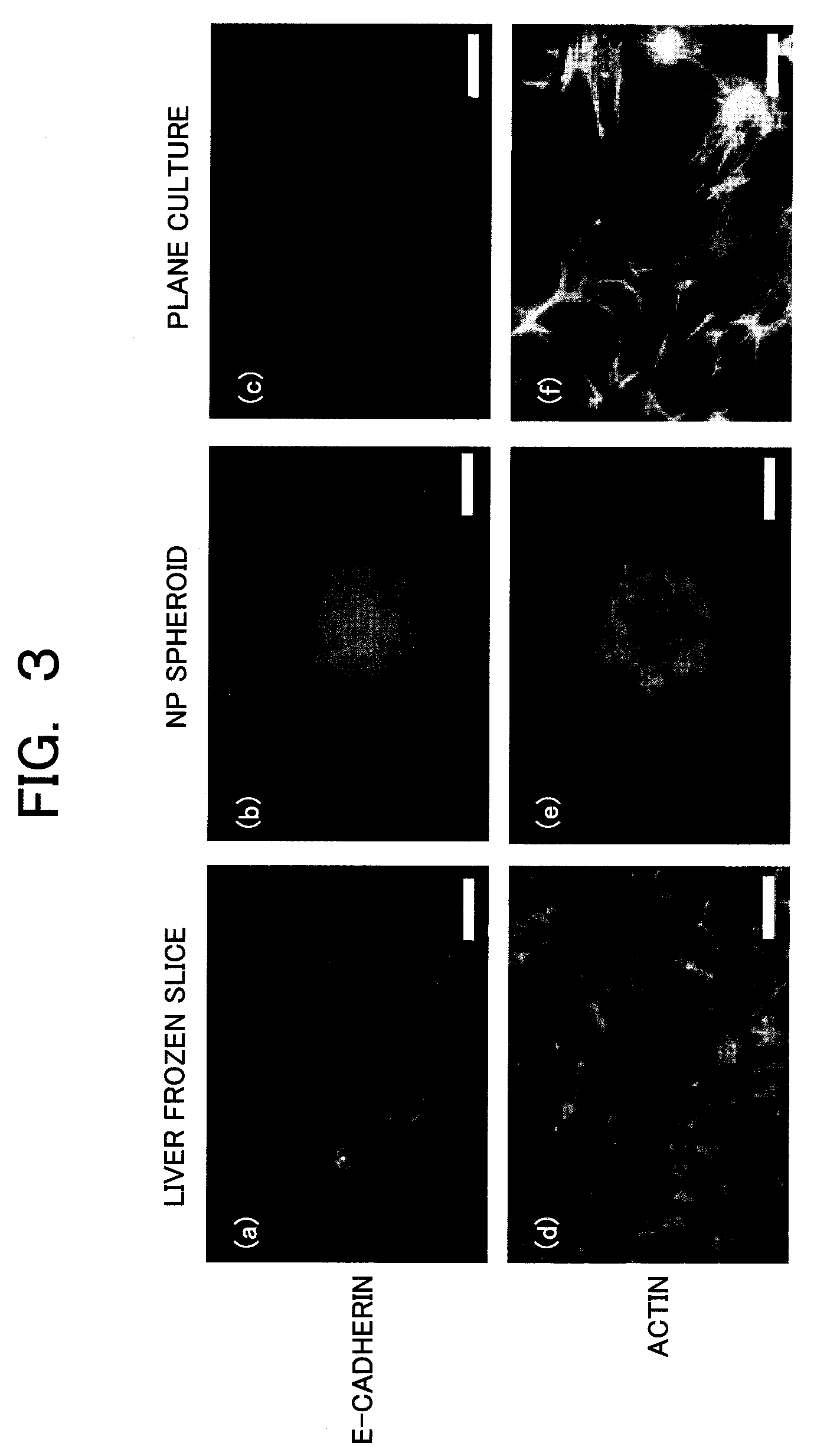
Provided are a technique for easily forming a spheroid by three-dimensionally culturing hepatocytes, and a technique for forming a spheroid having a higher expression level of a transporter MRP2 playing a role of biliary excretion than that of a conventional method. In order to solve the above-described problems, the present inventors have found out a condition under which hepatocytes easily form the spheroid on a nanopillar sheet. More specifically, this is related to a concentration of Type I collagen coated onto the NP sheet. Also, they have found out a condition under which an expression level of a gene related to the excretion of the formed spheroid is improved. More specifically, after the spheroid is previously formed, a biological matrix is overlayered thereon.
Owner:HITACHI LTD
Amide compound and preparation method, medicinal composition and application thereof
ActiveCN104292120ALower levelReduce cholesterolOrganic active ingredientsOrganic compound preparationMetaboliteBile Juice
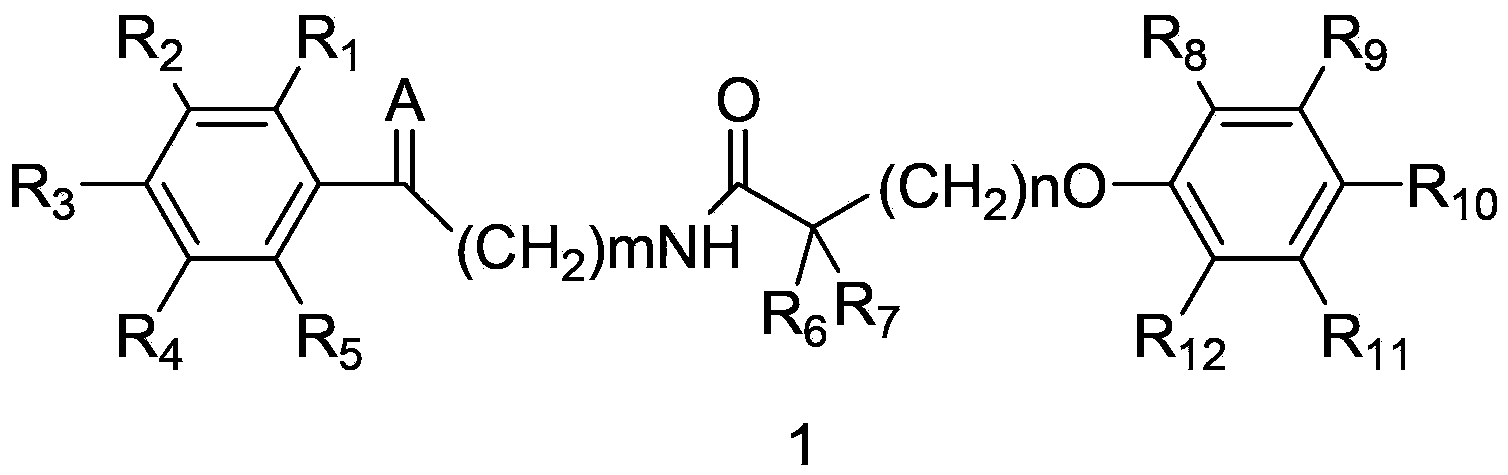
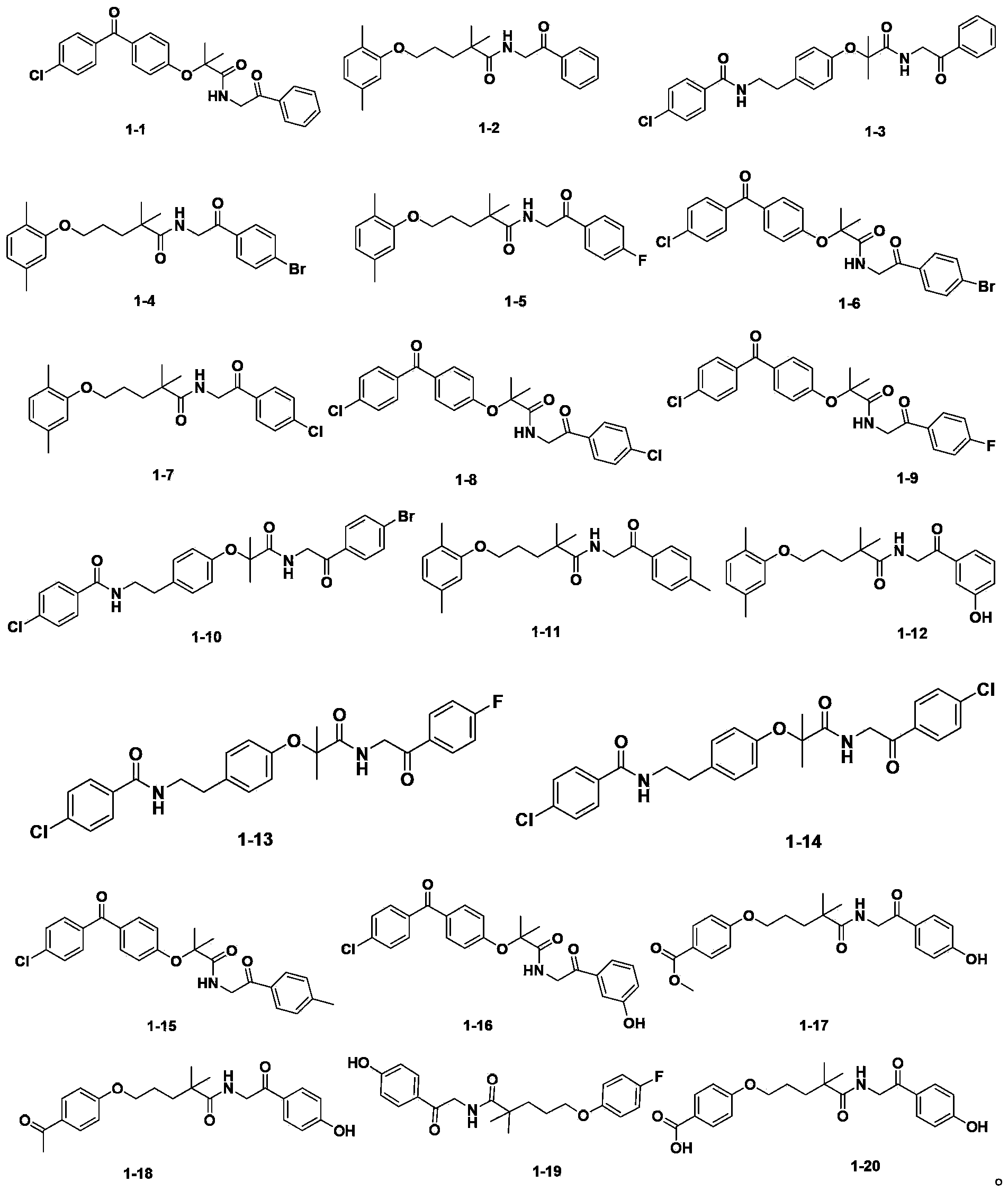
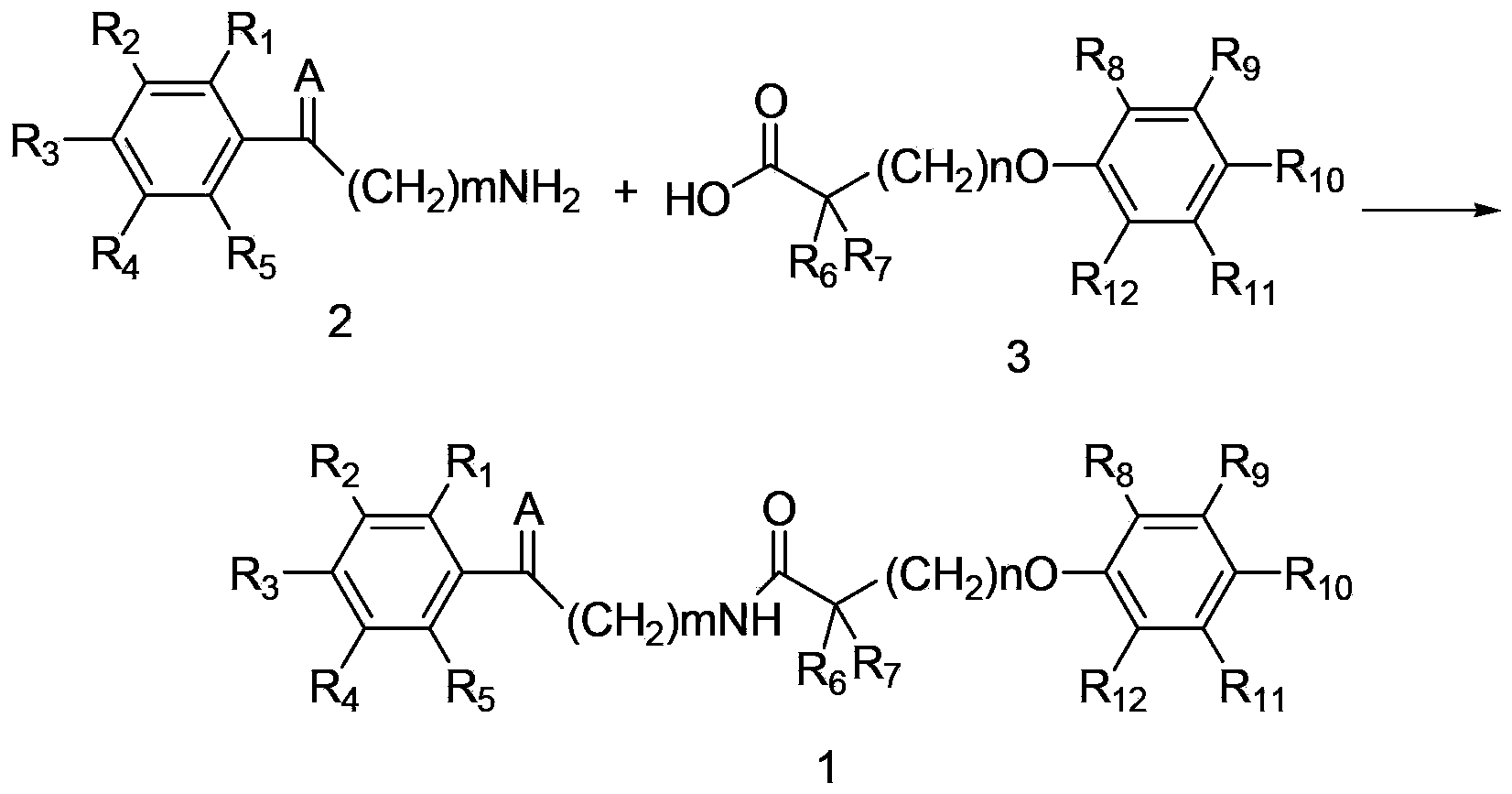
The invention discloses an amide compound and a preparation method, a medicinal composition and an application thereof. The invention provides an amide compound which is shown as a formula I, and a pharmaceutically-acceptable salt, a metabolite, a metabolism precursor or a prodrug thereof. The amide compound has the effects of reducing cholesterol and low density lipoprotein in blood, and also has the effects of promoting biliary excretion, lowering the content of cholesterol in bile, increasing the content of bile acid, and preventing and treating cholelithiasis.
Owner:SHANGHAI INST OF PHARMA IND CO LTD +1
Rabdosia lophanthide extractive, natural medicine for treating hepatitis
InactiveCN1405177ASimple manufacturing processClear antiviralOrganic active ingredientsOrganic compounds purification/separation/stabilisationBiliary excretionProthoracic gland
A kind of inartificial medicine Rabdosia.serra (Maxim) Hara extraction materials which may cure hepatitis and its preparation method is disclosed in the invention, which acts Rabdosia.serra (Maxim) Hara as materials, adopts one of the hot water, supercriticality CO2 or organic impregnant extracting methods, and executes Rabdosia.serra (Maxim) Hara extracting materials by decompression condensation, vacuum dryness, spray dryness etc. methods. It contains antivirus active component 2-hydroxyl ursocic acid, 2,3-di hydroxyl ursocic acid and antioxygenation active component rosmarinic acid and so on speciality valid components, and so it has restraining HBV copy, protecting immunity liver damnification, advancing cell immunity and increasing spleen, thymus weight, bile drainage of low immunity experiment animals etc. functions.
Owner:GUANGZHOU UNIVERSITY OF CHINESE MEDICINE
Method of screening a metabolite of a parent candidate compound for susceptibility to biliary excretion
InactiveUS7682781B2Inexpensive and rapid methodMore compoundedMicrobiological testing/measurementArtificial cell constructsMetaboliteBiliary excretion
A method of screening a candidate compound for susceptibility to biliary excretion. The method includes the steps of providing a culture of hepatocytes, the culture having at least one bile canaliculus; exposing a candidate compound to the culture; and determining an amount of candidate compound in the at least one bile canaliculus, the amount of candidate compound in the at least one bile canaliculus indicating the susceptibility of the candidate compound to biliary excretion. Optionally, the culture of hepatocytes is a long-term culture in a sandwich configuration. The method is particularly applicable to the screening of multiple candidate compounds in a single effort.
Owner:THE UNIV OF NORTH CAROLINA AT CHAPEL HILL
Composite fungal intercellular polysaccharide composition with liver protecting function and preparation method
InactiveCN104739896AReduce MDA contentResist damageDigestive systemFungi medical ingredientsBiliary excretionLiver necrosis
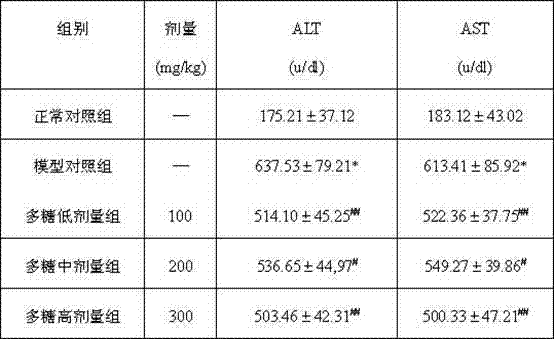
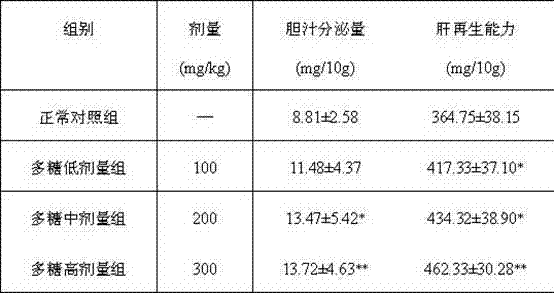
The invention discloses a composite fungal intercellular polysaccharide composition with a liver protecting function and a preparation method. The composite fungal intercellular polysaccharide composition comprises 10-45 weight parts of coprinus comatus intercellular polysaccharide, 20-50 weight parts of white fungus intercellular polysaccharide and 20-45 weight parts of hohenbuehelia serotina intercellular polysaccharide. The preparation method comprises the steps of fermenting fungi, crushing the separated mycelia, adding enzyme for reacting, and separating solid and liquid phases; adding distilled water into the solid phase, then performing ultrasonic treatment, adding ethanol liquid, collecting and drying the sediment; concentrating the liquid phase, adding ethanol, standing, centrifuging to separate the sediment; combining the sediments obtained by solid and liquid phase treatment to obtain homogenous intercellular polysaccharides; and mixing the coprinus comatus intercellular polysaccharide, the white fungus intercellular polysaccharide and the hohenbuehelia serotina intercellular polysaccharide obtained by the method respectively to obtain the composite fungal intercellular polysaccharide composition with the liver protecting function. The composition has the advantages of effectively reducing the ALT (alanine aminotransferase) and AST (aspartate aminotransferase) level of serum, obviously reducing the malonaldehyde content of injured liver and obviously promoting biliary excretion so that the function of the injured liver can be recovered.
Owner:JILIN MODERN TRADITIONAL CHINESE MEDICINE ENG RES CENT
Traditional Chinese medicine composition for control of laying hen fatty liver syndrome and preparation method thereof
InactiveCN104800746ASlow rotationWon't happenOrganic active ingredientsDigestive systemFatty liverBetaine
The invention relates to a composition for control of laying hen fatty liver syndrome and a preparation method thereof. The composition comprises the following components by weight: 20 parts of Alisma, 5-40 parts of the root of red-rooted salvia, 5-25 parts of radix bupleuri, 5-10 parts of turmeric, 1-2 parts of vitamin E and 1-2 parts of betaine. The composition provided by the invention has significant effects of promoting fat metabolism, inhibiting fat accumulation in the liver and increasing bile excretion, can effectively alleviate and reduce the incidence of fatty liver syndrome and improve egg production efficiency of laying hens.
Owner:TIANJIN JIACHUANG BIO TECH
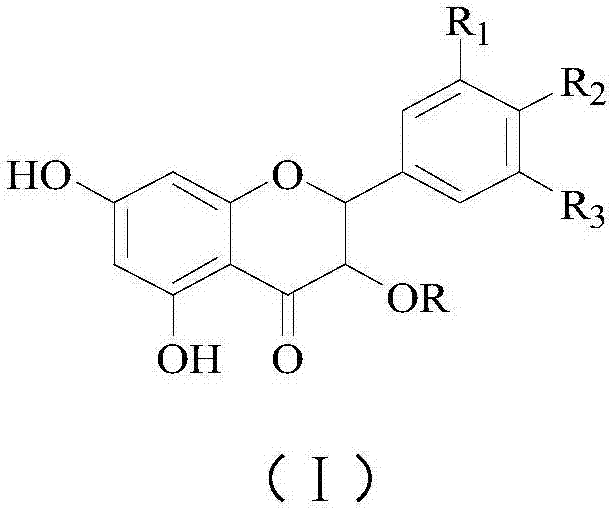
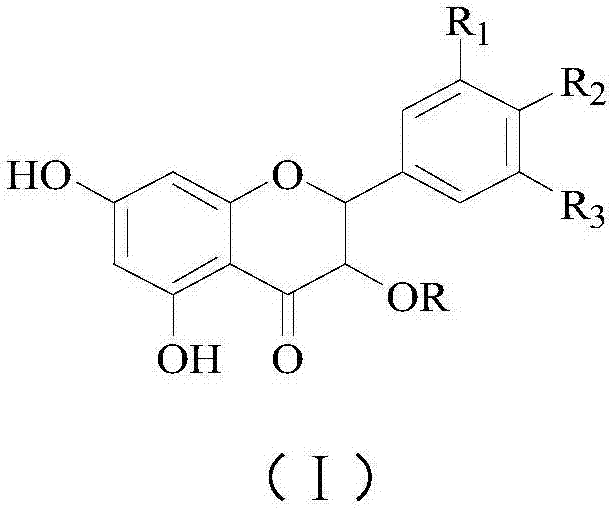
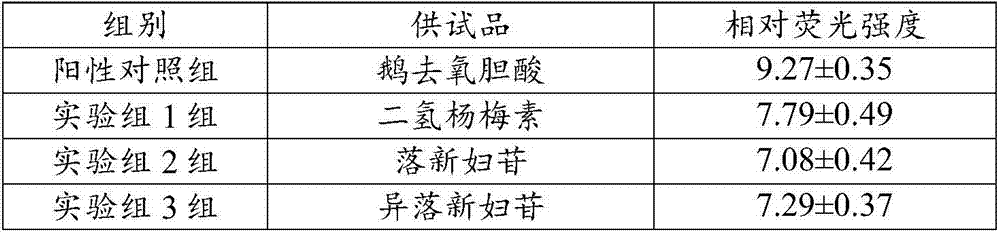
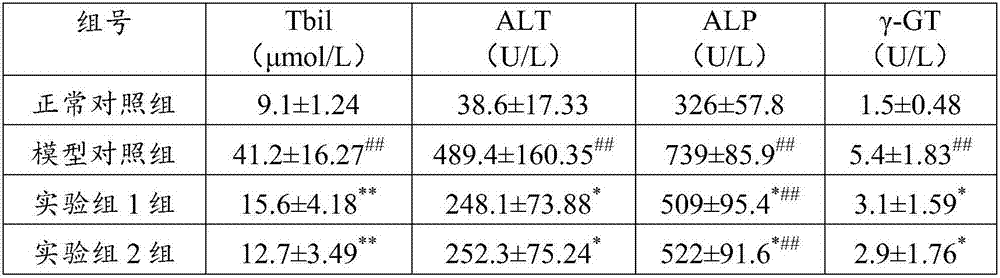
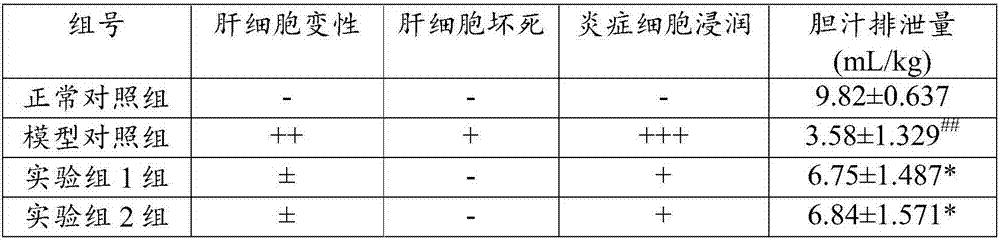
Application of flavonone compounds in preparation of farnesol X receptor stimulant
InactiveCN107397743AStrong FXR activationEasily damagedOrganic active ingredientsDigestive systemTreatment effectBiliary excretion
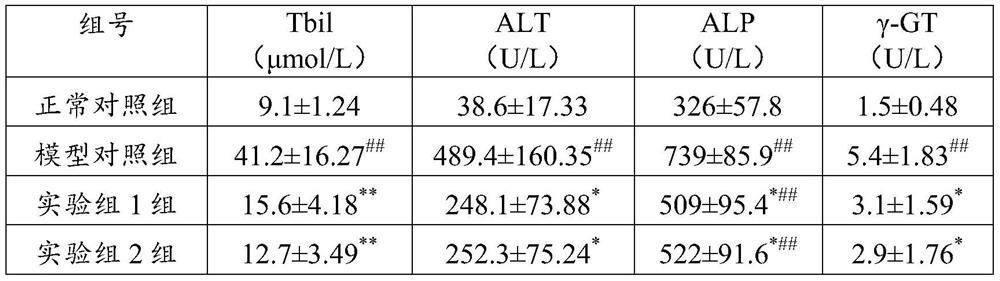
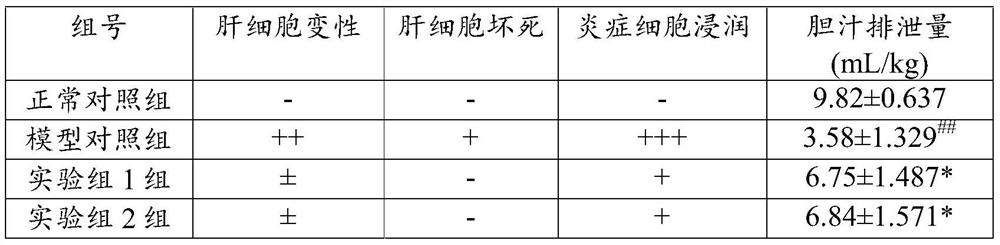
The invention relates to application of flavonone compounds in the preparation of a farnesol X receptor stimulant. The flavonone compounds including dihydromyricetin, astilbin, isoastilbin, engeletin, isoengeletin, dihydroquercetin and dihydrokaempferol have a relatively strong FXR stimulation effect and can be used as a potential farnesol X receptor stimulant. The flavonone compounds including dihydromyricetin, astilbin, isoastilbin, engeletin, isoengeletin, dihydroquercetin and dihydrokaempferol have a remarkable inhibition effect to the active increase of Tbil, ALT, APL and gamma-GT caused due to ANIT-induced cholestasis, are capable of obviously improving the inhibition to ANIT-induced hepatic cell injury, inflammatory cell infiltration and bile excretion, have a relatively good treatment effect to cholestasis type hepatitis and can be used as potential drugs for treating the cholestasis type hepatitis.
Owner:CATCH BIO SCI & TECH
Application of engelhardia roxburghiana extract in preparing farnesol X receptor agonist
ActiveCN107397785AHas FXR activationStrong activationDigestive systemPlant ingredientsBiliary excretionFarnesoid X Receptor Agonists
The invention relates to application of engelhardia roxburghiana extract in preparing a medicament for treating cholestatic liver disease. The engelhardia roxburghiana extract ER and ER-A2 have certain FXR activating action, and the engelhardia roxburghiana extract ER-A3 and ER-B2 have relatively strong activating action on FXR and can be directly used as a farnesol X receptor agonist. In addition, the engelhardia roxburghiana extract ER-A3 and ER-B1 prepared by the invention have remarkable inhibiting action on activity rise of Tbil, ALT, APL and gamma-GT caused by cholestasis induced by ANIT, obviously improve the hepatocyte damage, inflammatory cell infiltration and bile excretion caused by ANIT and have relatively good therapeutic action on cholestatic liver disease, and can be used as a potential drug for treating cholestatic liver disease.
Owner:CATCH BIO SCI & TECH
Compound preparation for treating cholestatic jaundice and preparing method thereof
InactiveCN105998052AAnti-inflammatoryCompatibility is reasonableDigestive systemEther/acetal active ingredientsTherapeutic effectBile fluid
The invention discloses a compound preparation for treating cholestatic jaundice and a preparing method thereof. The compound preparation is prepared from ademetionine 1,4-butanedisulfonate, diammonium glycyrrhizinate, dihydroxydibutylether, baicalin, methionine, anethol trithione, potassium magnesium aspartate, crocetin dimethyl ester, glucurolactone, cholestyramlne, hydroxymethylnicotinamide, trimethoprim lactate, bumetanide, piperazine citrate and azathioprine. The compound preparation has the effects of resisting bacteria and diminishing inflammations, clearing dampness and heat, soothing the liver and benefiting the gallbladder, removing the toxicity and eliminating jaundice and strengthening the spleen and the stomach, can reduce stasis of bile in liver cells, promote bile excretion, improve the liver functions and the tissue cell respiration function, reduce accumulation of fat in the liver and promote jaundice elimination and liver function recovery and is significant in treatment effect, safe and few in side effect when the compound preparation is used for treating the cholestatic jaundice and complications thereof.
Owner:徐海燕
Traditional Chinese medicine enema for treating hyperbilirubinemia of newborn and nursing method
InactiveCN104815174ABlock excretionDigestive systemPharmaceutical delivery mechanismBILIRUBINAEMIABiliary excretion
The invention discloses traditional Chinese medicine enema for treating hyperbilirubinemia of newborn. The hyperbilirubinemia of newborn belongs to traditional Chinese medicine fetal jaundice-newborn jaundice, and the theory of traditional Chinese medicine thinks that the newborn jaundice is caused by the weakness of the spleen and the stomach, dampness-heat fumigation or dampness and hotness obstruction and biliary excretion blockage of a fetus in the maternal body. The traditional Chinese medicine enema for treating the hyperbilirubinemia of newborn is prepared from traditional Chinese medicines comprising wintergreen barberry root, pilosella-leaved drymoglossum herb, japanese xylosma bark, senna, common clearweed herb, creeping rostellularia herb, mile swertia, smallflower epipactis herb, rust-coloured crotalaria herb with root and smallflower seaberry herb, and the traditional Chinese medicines are prepared into enema. The clinic test proves that each index is better than the control group, and the curative effect is obvious.
Owner:李英
Application of tripterine in preparation of medicines for treating cholestatic liver disease
ActiveCN106924265AReduce bile acid contentReduce necrosisOrganic active ingredientsDigestive systemCholic acidBiliary excretion
The invention discloses application of tripterine in preparation of medicines for treating cholestatic liver disease. The tripterine achieves obvious effects of improving cholestasis and alleviating liver damage on intrahepatic cholestasis induced by mouse alpha-naphthylisothiocyanate (ANIT) and cholestatic liver disease induced by thioacetamide (TAA), can obviously lower levels of aspartic transaminase (AST), alanine aminotransferase (ALT) and alkaline phosphatase (ALP) in plasma of two models, reduce the cholic acid content in the plasma and promote bile excretion and has the effects of protecting liver and increasing choleresis; and when being applied to research and development of medicines with effects of protecting liver and increasing choleresis, the tripterine can create excellent economic benefits and wide social benefits.
Owner:KUNMING INST OF BOTANY - CHINESE ACAD OF SCI
Traditional Chinese medicine oral liquid of couch grass root for treating chronic hepatitis and preparation method thereof
ActiveCN102688401AAvoid damageRecovery functionAnthropod material medical ingredientsDigestive systemBiliary excretionChronic hepatitis
The invention discloses a traditional Chinese medicine oral liquid of couch grass root for treating chronic hepatitis, comprising the following traditional Chinese medicine raw materials in parts by weight: 90-180 parts of couch grass root, 90-180 parts of virgate wormwood herb, 30-90 parts of largehead atractylodes rhizome, 30-90 parts of ginger pinellia, 30-90 parts of bupleurum, 90-180 parts of radix rehmanniae, 30-90 parts of pseudo-ginseng, 30-90 parts of earthworm and 30-60 parts of liquorice. The traditional Chinese medicine preparation disclosed by the invention can eliminate qi movement stasis of liver and gall, alleviate harm to liver, normalize the function of choleresis and biliary excretion and take the effects of strengthening the body resistance without stasis of pathogenic factor and eliminating pathogenic factor without hurt to the body resistance. The oral liquid has low cost and is worthy of clinical popularization and application.
Owner:南通海门凤成旅游文化发展有限公司
Method for culturing animal hepatocyte
Provided are a technique for easily forming a spheroid by three-dimensionally culturing hepatocytes, and a technique for forming a spheroid having a higher expression level of a transporter MRP2 playing a role of biliary excretion than that of a conventional method. In order to solve the above-described problems, the present inventors have found out a condition under which hepatocytes easily form the spheroid on a nanopillar sheet. More specifically, this is related to a concentration of Type I collagen coated onto the NP sheet. Also, they have found out a condition under which an expression level of a gene related to the excretion of the formed spheroid is improved. More specifically, after the spheroid is previously formed, a biological matrix is overlayered thereon.
Owner:HITACHI LTD
Liver soothing and choleresis increasing lipid-lowering tea
InactiveCN106922893AImprove stasisImprove stickinessDispersion deliveryMetabolism disorderLiver functionsCellulose
The invention relates to the technical field of health care products, in particular to a liver-soothing and gallbladder-reducing lipid-lowering tea, which is made of the following raw materials in parts by mass: 20-30 parts of turmeric, 20-30 parts of Bupleurum, and 18-28 parts of Citrus aurantii , 18-28 parts of peony, 16-24 parts of tangerine peel, 16-24 parts of black wolfberry, 14-20 parts of cassia seed, 14-20 parts of Cyperus cyperi, 10-20 parts of astragalus, 5-15 parts of gallinaceous gold, 5- 15 parts, 5-15 parts of light bamboo leaves, 5-15 parts of kudzu root, 5-7 parts of licorice, 5-7 parts of pinellia, 5-7 parts of woody fragrance, 5-7 parts of money grass, 3-5 parts of Andrographis paniculata, chrysanthemum 1-3 parts, malt 1-3 parts. The liver-soothing and gallbladder-reducing lipid-lowering tea of the present invention can improve the stasis and thick viscosity of blood and bile, improve liver function, promote bile excretion, thereby reducing blood fat; in addition, it contains rich cellulose, pectin, etc., which can reduce cholesterol Absorption, and further play a role in lowering blood lipids.
Owner:张培君
Traditional Chinese medicine for treating chololithiasis
InactiveCN105343202APromote excretionEasy to shrinkPowder deliveryAnthropod material medical ingredientsMedicinal herbsBile Juice
The invention relates to a traditional Chinese medicine and provides a traditional Chinese medicine for treating chololithiasis. The traditional Chinese medicine is characterized by comprising medicinal materials in parts by weight as follows: 3-12 parts of rhubarb, 3-15 parts of mirabilite, 6-20 parts of giant knotweed rhizomes, 3-15 parts of chicken's gizzard-skin, 3-15 parts of maggots, 3-12 parts of aluminite, 3-15 parts of earthworms, 3-15 parts of dung beetles and 3-15 parts of mole crickets. According to the traditional Chinese medicine, the rhubarb and the mirabilite are selected for relaxing bowels, diminishing inflammations, keeping the bowels open and promoting waste excretion; the giant knotweed rhizomes, the chicken's gizzard-skin and the maggots are selected for promoting bile excretion and removing stagnation; the aluminite, the earthworms, the dung beetles and the mole crickets are selected for removing and dissolving stone; all the medicinal materials cooperate to play effects of promoting gallbladder contraction, expanding bile ducts, increasing bile secretion and excretion, dissolving the stone and removing the stone so as to treat the chololithiasis.
Owner:邓瑞萍
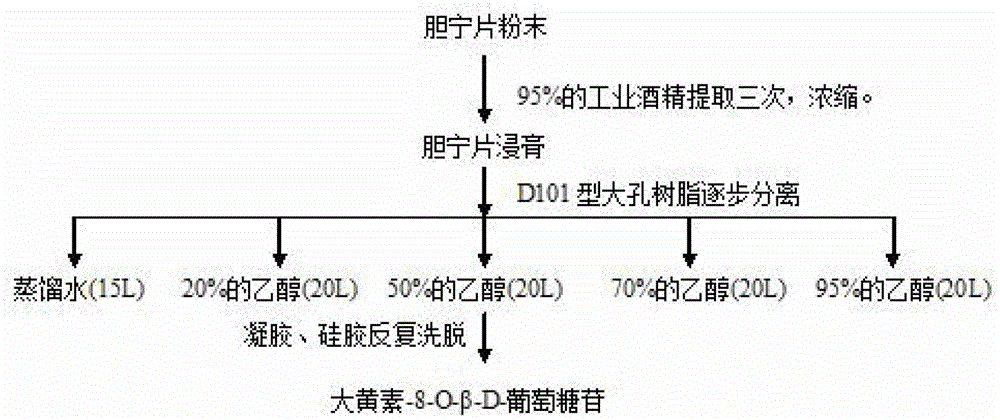
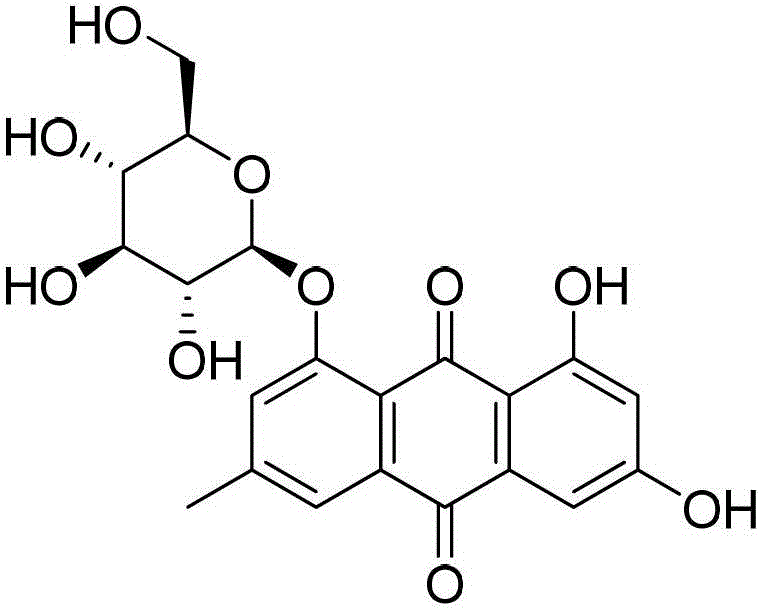
New application of emodin-8-O-beta-D-glucoside in preparation of liver-protecting and gallbladder-benefiting medicaments
ActiveCN103156873BPromote excretionHigh trafficOrganic active ingredientsSugar derivativesDiseaseBiliary excretion
The invention relates to the field of research of traditional Chinese medicine and specifically relates to a new application of emodin-8-O-beta-D-glucoside in preparation of liver-protecting and gallbladder-benefiting medicaments. The invention further prepares a liver-protecting and gallbladder-benefiting preparation and the active ingredient of the liver-protecting and gallbladder-benefiting preparation is emodin-8-O-beta-D-glucoside. According to the new application disclosed by the invention, research based on an ANIT (alpha-naphthylisothi)-induced intrahepatic cholestasis model of a rat proves that emodin-8-O-beta-D-glucoside is capable of significantly reducing serum transaminase level, promoting bile excretion, increasing the flow rate of bile and realizing liver-protecting and gallbladder-benefiting effects on intrahepatic cholestasis diseases, and great economic benefits and extensive social benefits can be created by application of the emodin-8-O-beta-D-glucoside to research and development of the liver-protecting and gallbladder-benefiting medicaments.
Owner:HEHUANG PHARMA SHANGHAI
Fluorescent probe for detecting glucuronyl transferase 1A1 and application thereof
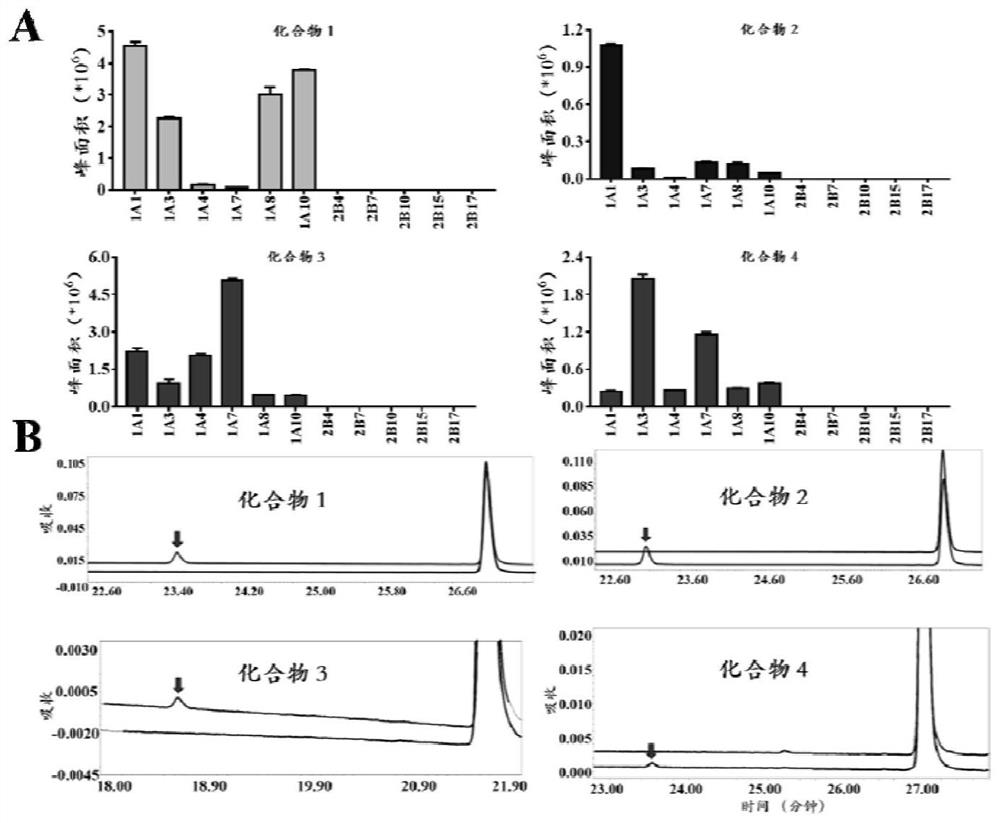
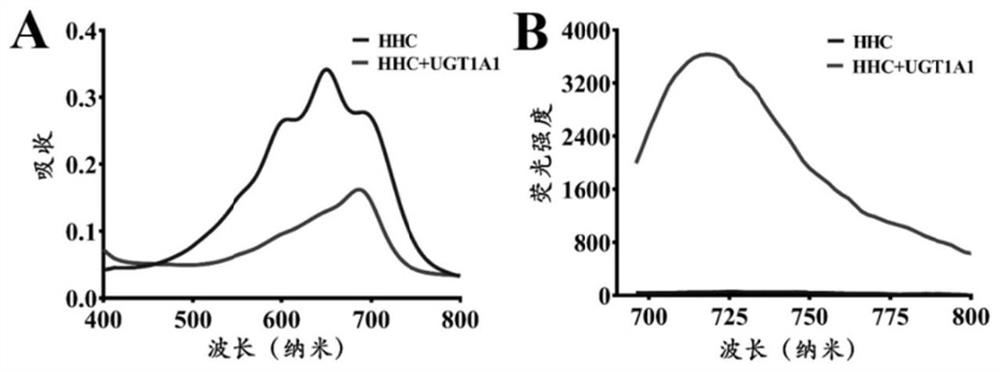
ActiveCN113307799AEasy accessGood cellsOrganic active ingredientsOrganic chemistryFluoProbesBiliary excretion
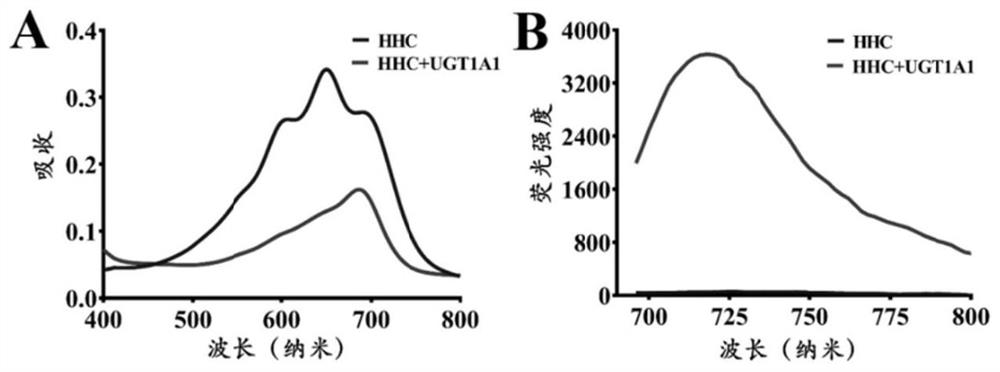
The invention discloses a fluorescent probe for detecting glucuronyl transferase 1A1 and application thereof, and belongs to the technical field of biological medicines. The fluorescent probe can be used for determining the activity of UGT1A1 in biological systems of different sources. HHC is used as a specific probe reaction substrate, by means of a glucuronyl transferase in-vitro reaction system, glucuronylation binding reaction of 5-site hydroxyl of HHC is used as a probe reaction, and the activity of UGT1A1 in each biological sample is determined by quantitatively detecting the generation amount of a metabolite HHC-5-beta-D-Glucuronoside (HHC-G) in unit time. The fluorescent probe can be used for qualitative and quantitative determination of UGT1A1 activity in different individual source human and animal tissue samples, different species of cells and cell preparations, and various plants and microorganisms, can also realize in-vivo imaging research on UGT1A1, and can rapidly evaluate the liver bile excretion function at the animal level. By evaluating the activity of UGT1A1 of different individuals, the method is used for guiding reasonable use of clinical drugs such as irinotecan and high-throughput screening of UGT1A1 inhibitors.
Owner:DALIAN MEDICAL UNIVERSITY
Traditional Chinese medicine oral liquid of couch grass root for treating chronic hepatitis and preparation method thereof
ActiveCN102688401BAvoid damageRecovery functionAnthropod material medical ingredientsDigestive systemBiliary excretionPinellia
Owner:南通海门凤成旅游文化发展有限公司
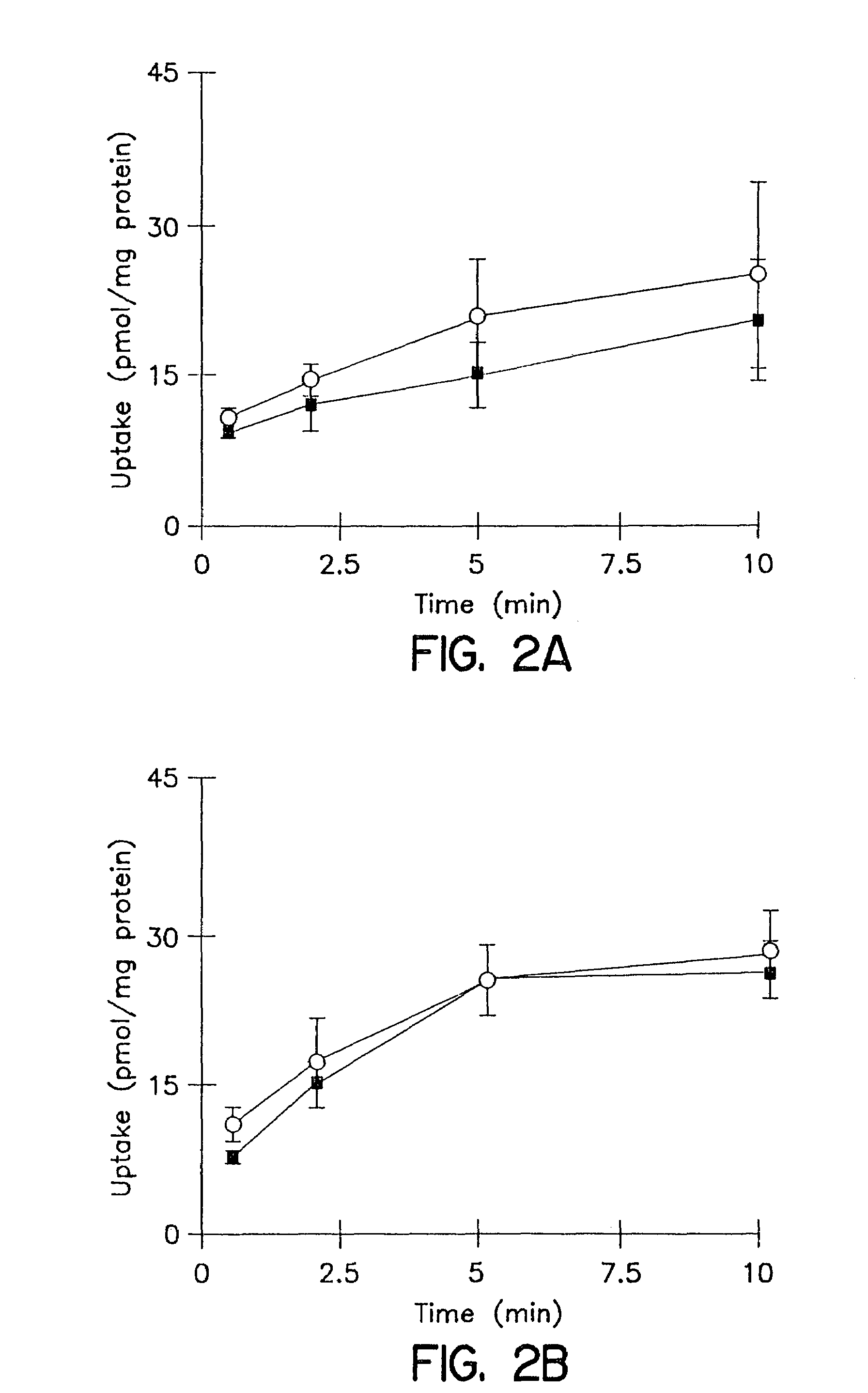
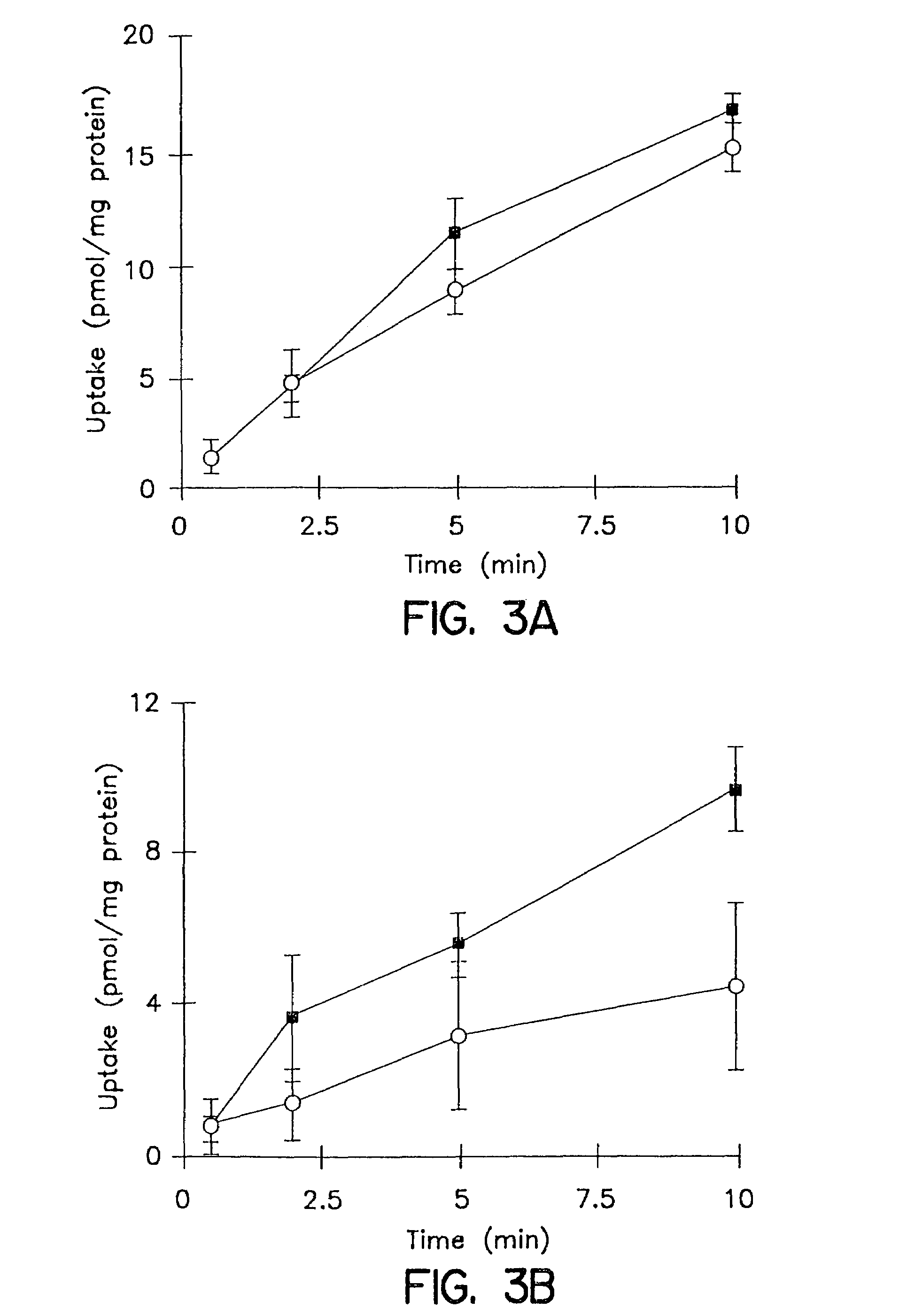
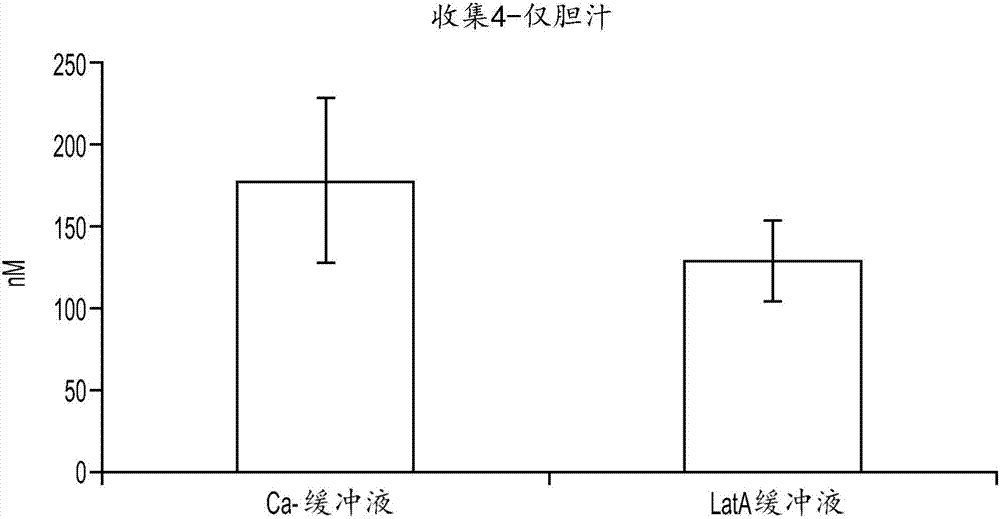
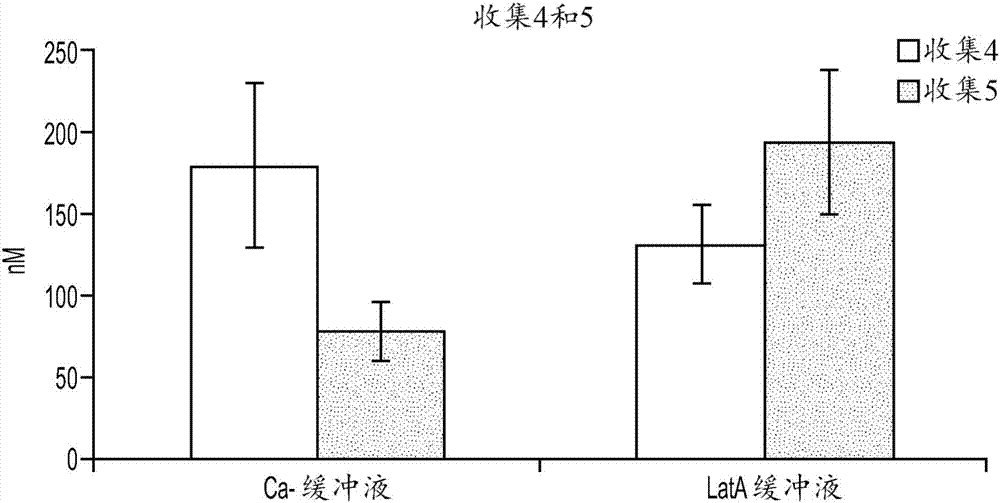
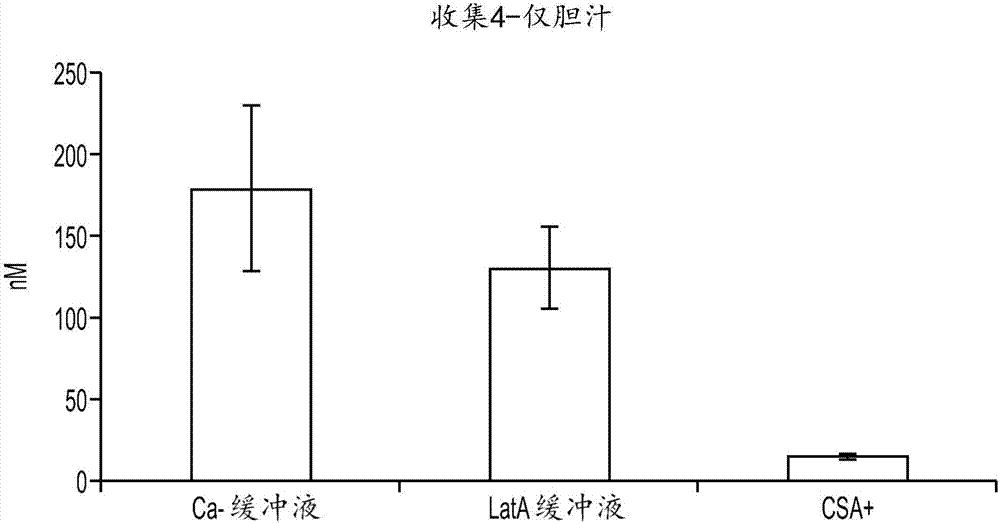
In vitro biliary excretion assay
InactiveUS20160061820A1Inhibitory activityMicrobiological testing/measurementBiological testingMetaboliteSufficient time
An in vitro methods of characterizing biliary excretion of a chemical entity using a single hepatocyte culture. Comprising providing cell culture comprising hepatocytes forming at least one bile canaliculus; contacting the cell culture with a first chemical entity for a time sufficient to allow uptake of the chemical entity by hepatocytes in the culture; disrupting the at least one bile canaliculus without lysing the hepatocytes and detecting the amount (if any) of the first chemical entity and / or a metabolite thereof released by the at least one bile canaliculus; and lysing the hepatocytes and detecting the amount of the first chemical entity and / or a metabolite thereof released by the hepatocytes.
Owner:HUREL CORP
In vitro biliary excretion assay
An in vitro methods of characterizing biliary excretion of a chemical entity using a single hepatocyte culture. Comprising providing cell culture comprising hepatocytes forming at least one bile canaliculus; contacting the cell culture with a first chemical entity for a time sufficient to allow uptake of the chemical entity by hepatocytes in the culture; disrupting the at least one bile canaliculus without lysing the hepatocytes and detecting the amount (if any) of the first chemical entity and / or a metabolite thereof released by the at least one bile canaliculus; and lysing the hepatocytes and detecting the amount of the first chemical entity and / or a metabolite thereof released by the hepatocytes.
Owner:HUREL CORP
A fluorescent probe for detecting glucuronyltransferase 1a1 and its application
ActiveCN113307799BEasy accessGood tissue permeabilityOrganic active ingredientsOrganic chemistryMetaboliteBiliary excretion
A fluorescent probe for detecting glucuronyl transferase 1A1 and its application belong to the technical field of biomedicine. It can be used to measure the activity of UGT1A1 in biological systems of different origins. Using HHC as the specific probe reaction substrate, with the help of the glucuronyl transferase in vitro reaction system, the glucuronidation binding reaction of the 5-hydroxyl group of HHC is used as the probe reaction, and the metabolite HHC-5- in unit time is quantitatively detected. β‑ The production of D-Glucuronoside (HHC-G) was used to measure the activity of UGT1A1 in each biological sample. The invention can be used for qualitative and quantitative determination of UGT1A1 activity in human and animal tissue samples from different individual sources, cells of different species and cell preparations, as well as various plants and microorganisms, and can also realize in vivo imaging research on UGT1A1. A rapid assessment of hepatic bile excretion function. By evaluating the activity of UGT1A1 in different individuals, it can be used to guide the rational use of clinical drugs, such as irinotecan, and high-throughput screening of UGT1A1 inhibitors.
Owner:DALIAN MEDICAL UNIVERSITY
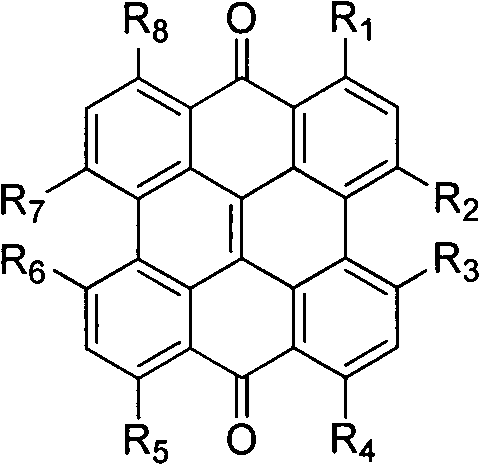
Naphthdianthrone compound and application of salt thereof in preparing developer of biliary tract and gall stones
InactiveCN102526766AEasy to identifyEasy to findIn-vivo testing preparationsFluorescenceBiliary excretion
The invention relates to a fluorescent developer, in particular to naphthdianthrone and salt thereof, which are injected intravenously or taken orally and displays the biliary tract and gall stones in operation. After being injected intravenously or taken orally, the developer is taken by hepatic cells, is discharged along with bile, is firmly combined with the gall stones and generates fluorescence under the excitation of certain wavelength light. The naphthdianthrone and salt thereof and developer provided by the invention are convenient for identifying the biliary tract structure, finding the intrahepatic and extrahepatic biliary duct stone and monitoring biliary tract damage and bile leakage in open surgery and peritoneoscope and choledochoscope surgeries.
Owner:JIANGSU PROVINCE INST OF TRADITIONAL CHINESE MEDICINE
In vitro biliary excretion assay
InactiveCN107208050AInhibitory activityMicrobiological testing/measurementVertebrate cellsMetaboliteBiliary excretion
Owner:HUREL CORP
Chinese medicinal herb pills for treating cholecystitis and gallstone
InactiveCN102526616BAlleviate inflammatory lesionsEasy to shrinkDigestive systemPill deliveryMedicinal herbsBile Juice
The invention relates to Chinese medicinal herb pills for treating cholecystitis and gallstone. The Chinese medicinal herb pills are prepared from 22 gouts of Chinese medicinal herbs, namely coptis root, baikal skullcap root, phellodendron, corydalis tuber, curcuma, costus root, loosestrife herb, Japanese climbing fern spore, dark plum, white peony root, fine-leaf schizonepeta herb, green tangerine peel, immature bitter orange, turmeric, polygonum cuspidatum, Szechwan chinaberry fruit, gentian, bupleurum, oriental wormwood, chicken's gizzard-membrane, compound of glauber-salt and liquorice and slaked lime. The pills can lighten the pathological change of cholecystitis, promote gallbladder contraction and promote gallstone excretion and biliary excretion; by the pills, biliary excretion components are radically changed, the metabolism requirement of the human body is met, calculi are gradually disintegrated and deposited, the micro environment in the gall bladder is recovered when the calculi are discharged, and the aim of thoroughly discharging the calculi is fulfilled. The Chinese medicinal herb pills have the advantages of quick response, short treatment course, high healing rate, low recurrence rate, treatment for both symptoms and root causes, no toxic or side effects and the like.
Owner:马金桥
Application of Huangqi Extract in Preparation of Farnesoid X Receptor Agonist
ActiveCN107397785BHas FXR activationStrong activationDigestive systemPlant ingredientsHepatic inflammationBile Juice
The invention relates to the application of Huang Qi extract in the preparation of medicine for treating cholestatic liver disease. The Huangqi extract ER and ER-A2 prepared by the present invention have a certain FXR activation effect, and the Huangqi extract ER-A3 and ER-B1 have a stronger activation effect on FXR, and can be used as a potential farnesoid X receptor stimulator In addition, the Huangqi extract ER-A3 and ER-B1 prepared by the present invention have a significant inhibitory effect on the increase in Tbil, ALT, APL and γ-GT activity caused by ANIT-induced cholestasis, and have a significant inhibitory effect on the cholestasis induced by ANIT. The liver cell injury, inflammatory cell infiltration and inhibition of bile excretion have been significantly improved, cholestatic hepatitis has a good therapeutic effect, and can be used as a potential drug for the treatment of cholestatic liver disease.
Owner:CATCH BIO SCI & TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com