Compound preparation for treating cholestatic jaundice and preparing method thereof
A compound preparation and cholestasis technology, which is applied in the direction of medical preparations containing active ingredients, pharmaceutical formulas, organic active ingredients, etc., can solve problems such as ineffective therapeutic effects, restore normal physiological functions, improve liver function, and reduce levels of Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
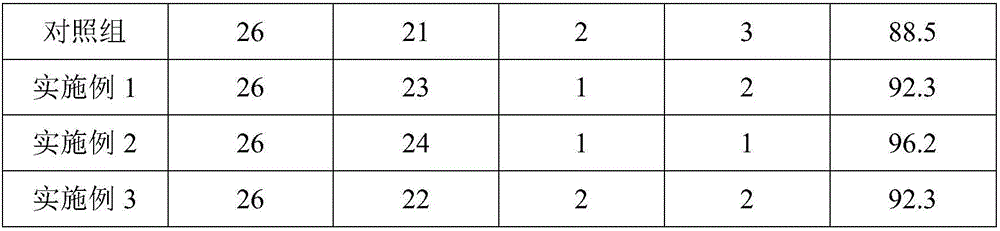
Embodiment 1
[0016] Example 1: This example prepares a compound capsule for the treatment of cholestatic jaundice, which is composed of the following components by weight: 57 parts of adenosylmethionine butanedisulfonate, 49 parts of diammonium glycyrrhizinate, 42 parts of dihydroxy dibutyl ether, 38 parts of baicalin, 27 parts of methionine, 22 parts of anetthio, 17 parts of potassium magnesium aspartate, 14 parts of dimethyl crocetin, 12 parts of glucuronolactone, 9 parts of cholestyramine, 3 parts of nicotinamide, 1 part of trimethoprim lactate, 1 part of bumetanide, 0.6 part of piperazine citrate, and 0.1 part of azathioprine.
[0017] Among them, the dihydroxy dibutyl ether is a colorless and transparent compound synthesized by using 1,3-butanediol as a raw material, using sulfonamide resin as a catalyst, and reacting for 2 hours at 140°C with the amount of the catalyst being 5% of the amount of the raw material. of oily liquid. Dihydroxydibutyl ether can promote the rapid, strong an...
Embodiment 2
[0025] Example 2: This example prepares a compound capsule for the treatment of cholestatic jaundice, which is composed of the following components by weight: 65.5 parts of adenosylmethionine butanedisulfonate, 56 parts of diammonium glycyrrhizinate, 46.5 parts of dihydroxydibutyl ether, 41.5 parts of baicalin, 31.5 parts of methionine, 26.5 parts of anetis trisulfide, 21 parts of potassium magnesium aspartate, 16.5 parts of dimethyl crocetin, 14.5 parts of glucuronolactone, Cholestyramine 11 parts, hydroxymethyl nicotinamide 5 parts, trimethoprim lactate 3 parts, bumetanide 2 parts, piperazine citrate 0.8 parts, azathioprine 0.3 parts.
[0026] Among them, the dihydroxy dibutyl ether is a colorless product synthesized by using 1,3-butanediol as raw material and sulfonamide resin as a catalyst at 140°C with the catalyst amount being 5% of the raw material amount and reacting for 2.5 hours. Transparent oily liquid. Dihydroxydibutyl ether can promote the rapid, strong and persi...
Embodiment 3
[0034] Example 3: This example prepares a compound capsule for the treatment of cholestatic jaundice, which is composed of the following components by weight: 74 parts of adenosylmethionine butanedisulfonate, 63 parts of diammonium glycyrrhizinate, 51 parts of dihydroxy dibutyl ether, 45 parts of baicalin, 36 parts of methionine, 33 parts of anetis trisulfide, 25 parts of potassium magnesium aspartate, 19 parts of dimethyl crocetin, 17 parts of glucuronolactone, Cholestyramine 13 parts, hydroxymethyl nicotinamide 7 parts, trimethoprim lactate 5 parts, bumetanide 3 parts, piperazine citrate 1 part, azathioprine 0.5 parts.
[0035] Among them, the dihydroxy dibutyl ether is a colorless and transparent compound synthesized by using 1,3-butanediol as raw material and sulfonamide resin as a catalyst at 140°C with a catalyst amount of 5% of the raw material amount and reacting for 3 hours. of oily liquid. Dihydroxydibutyl ether can promote the rapid, strong and persistent secretion...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com