Naphthdianthrone compound and application of salt thereof in preparing developer of biliary tract and gall stones
A naphthodianthrone and compound technology, applied in preparations for in vivo tests, pharmaceutical formulations, etc., can solve the problem of non-specific affinity for gallstones, no gallstones, no biliary tract and gallstone imaging agents And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
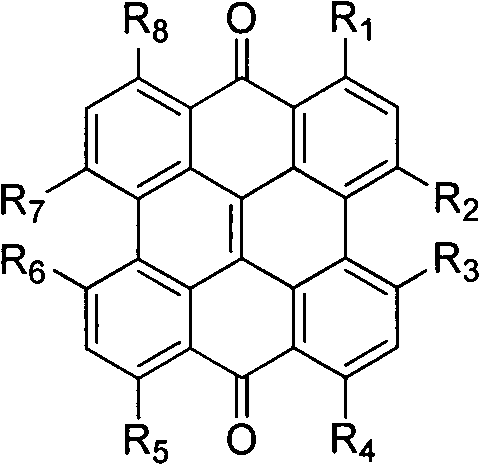
[0018] Reagents: SD rats were purchased from Shanghai Slack Experimental Animal Co., Ltd.; hypericin, pseudohypericin, trumpetin, hydroxyanthraquinone, nude pigment B, nude pigment D, heteronuclein D and chrysanthemum Purple was purchased from Beijing Xiantong Times Pharmaceutical Technology Development Co., Ltd.
[0019] Preparation preparation method: Weigh the above naphthodianthrone compounds and dissolve them in an appropriate amount of mixed solvent DMSO: PEG400: propylene glycol: normal saline (1:1:1:1), and configure them to be 0.1, 1.0, 5.0, and 20.0 mg respectively / ml solution.
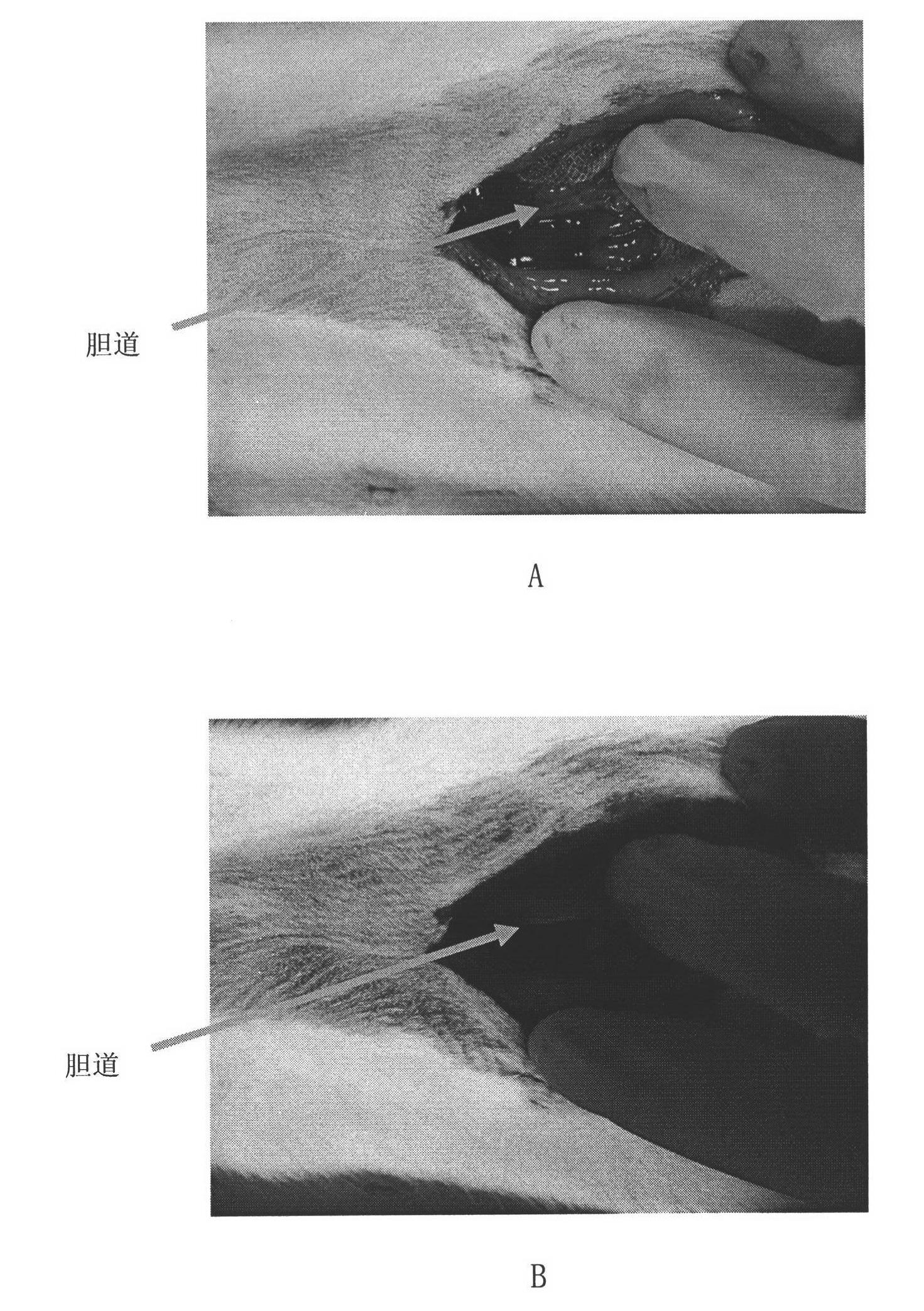
[0020] Experimental method: 108 SD male rats were divided into 36 groups, including hypericin, protohypericin, pseudohypericin, trumpetin, hydroxyanthraquinone, nude pigment B, nude pigment D, iso 0.1, 1.0, 5.0, 20.0 mg / ml dosage groups of nude pigment D and ocher violet. Pentobarbital anesthetized, fixed, opened the abdomen to expose the bile duct, intravenous injection of different dose...
Embodiment 2
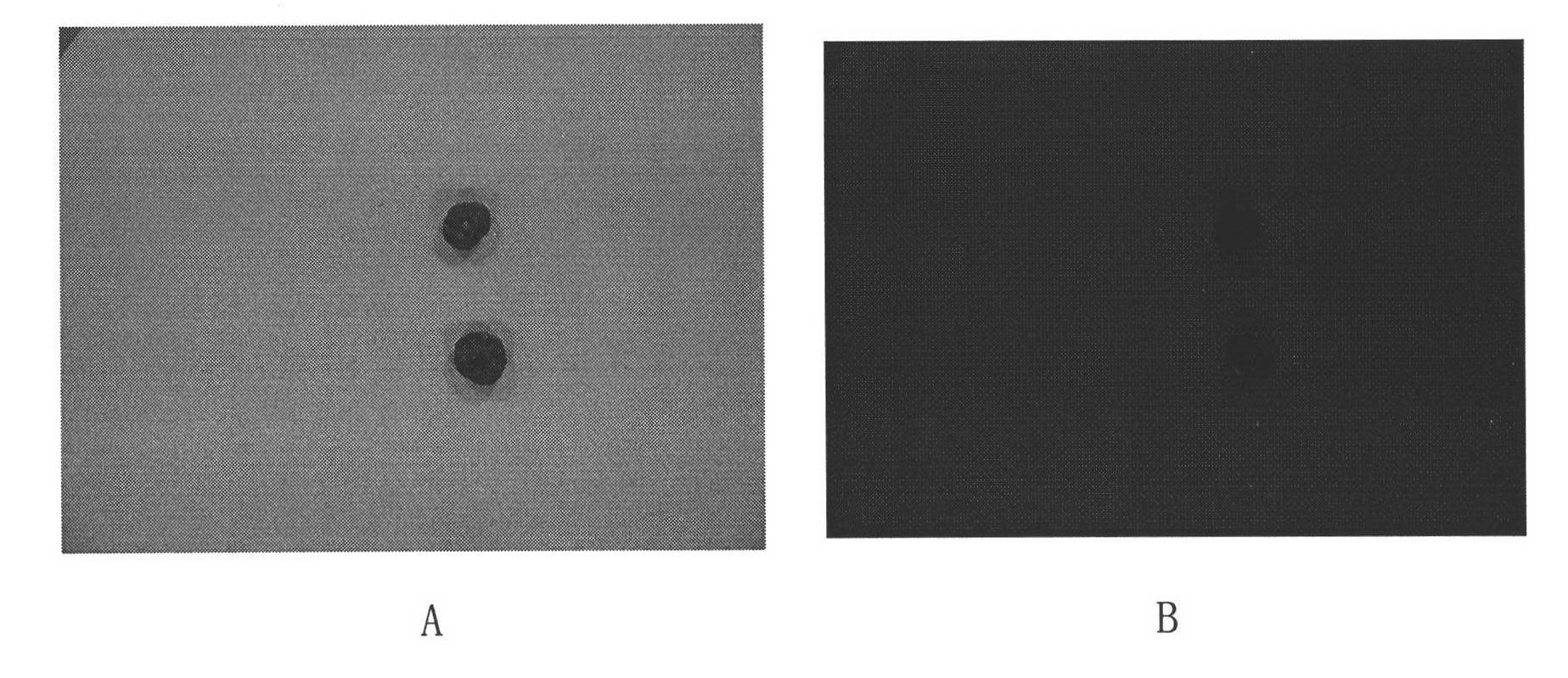
[0022] Prepare different concentration solutions of hypericin, pseudohypericin, trumpetin, hydroxyanthraquinone, nude pigment B, nude pigment D, isonuclein D and ocher violet, each concentration is 0.002, 0.02, 0.2 , 2mg / ml, human gallstones were taken, 6 human gallstones were put into solutions of different concentrations of each compound, and 6 pieces were put into blank bile, taken out after 24 hours, washed with bile and distilled water in turn, and placed under ultraviolet light at 365nm The fluorescence was observed, and the results showed that the gallstones in the bile solution of the above compound had red fluorescence, and the gallstones in the blank bile had no fluorescence.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com