A fluorescent probe for detecting glucuronyltransferase 1a1 and its application
A technology for glucuronic acid and fluorescent probes is applied in the field of detecting glucuronyltransferase 1A1 fluorescent probes, which can solve the problems of low efficiency, poor stability, and complicated detection, and achieve the effects of high efficiency, high speed and low noise.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] Example 1. Confirmation of the structure of the dihydroxy probe
[0070] (1) Determination of the optimal fluorescent site in the probe
[0071]
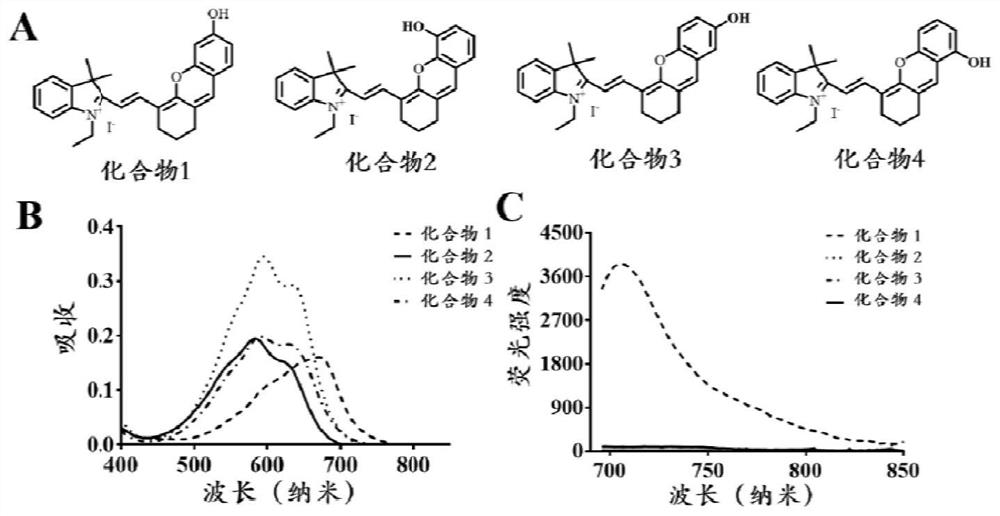
[0072] Hydroxyl-substituted compounds 1-4, wherein the hydroxy group in compound 1 is substituted at the 6-position, the hydroxyl group in compound 2 is substituted at the 5-position, the hydroxyl group in compound 3 is substituted at the 7-position, and the hydroxyl group in compound 4 is substituted at the 8-position.
[0073] The absorption spectrum and fluorescence spectrum of compound 1-4 were measured. The absorption spectrum of compound 1-4 in Tris-HCl system was collected at 400-850 nm; the fluorescence emission spectrum of compound 1-4 in Tris-HCl system, excitation wavelength 670nm, acquisition band 696-850nm. The result is as figure 1 As shown, the maximum absorption of compound 1 is at 690 nm, while the maximum absorption of compounds 2-4 is around 580 nm; meanwhile, the fluorescence spectrum shows that only ...
Embodiment 2
[0077] The synthesis of embodiment 2 compound HHC
[0078] The synthetic route of HHC is as follows:
[0079]
[0080] 3,4-Dimethoxy-2-hydroxybenzaldehyde (1 mmol) and 2-bromo-1-cyclohexene-1-carbaldehyde (284 mg, 1.5 mmol) were dissolved in 10 mL of N,N-dimethyl In the formamide, cesium carbonate (815 mg, 2.5 mmol) was added and stirred at room temperature for 2 h. Then, the reaction solution was poured into deionized water, extracted with ethyl acetate, the organic phase was concentrated by distillation under reduced pressure, and the residue was separated by column chromatography (petroleum ether:ethyl acetate=3:1) to obtain a yellow solid. 139mg, yield 51%. 1 H NMR (400MHz, CDCl 3 )δ10.44(s,1H),6.88(d,J=8.5Hz,1H),6.67(d,J=8.5Hz,1H),6.64(s,1H),3.92(s,3H),3.90( s, 3H), 2.58(dd, J=8.8, 3.6Hz, 2H), 2.46(t, J=6.1Hz, 2H), 1.76–1.70(m, 2H).
[0081] Compound 3 (0.1 mole) and compound 4 (32 mg, 0.1 mmol) were dissolved in 2 mL of acetic anhydride, potassium carbonate (21 m...
Embodiment 3
[0083] Example 3 In vitro determination of the selectivity of HHC to different UGTs single enzymes
[0084] (1) Prepare 190 μL of in vitro metabolic reaction system in advance, including Tris-HCl buffer (50 mM) at pH 7.4, different types of UGT single enzymes (0.1 mg / mL), HHC (final concentration 10 μM), and pre-incubate with shaking at 37 °C 3 minutes;
[0085] (2) Add 10 μL of UDPGA at a concentration of 40 mM (final concentration of 2 mM) to the reaction system to initiate the reaction;
[0086] (3) After 30 minutes, 100 μL of ice acetonitrile was added, and the reaction was terminated after vigorous shaking;
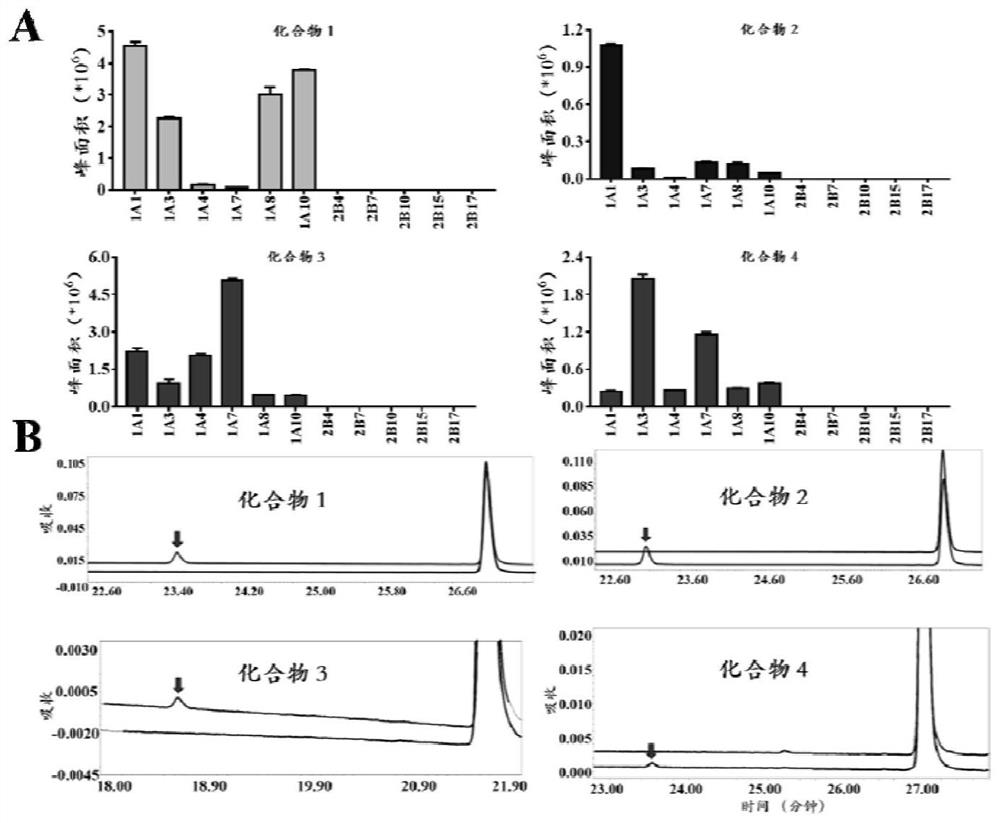
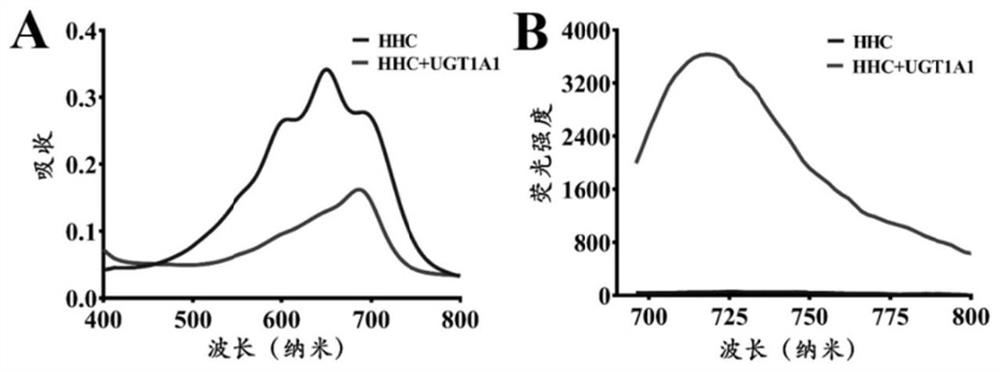
[0087] (4) Using a high-speed refrigerated centrifuge at 4°C and 20,000×g, after high-speed centrifugation for 20 minutes, take the supernatant for fluorescence detection (HHC-G: Ex=670nm, Em=720nm, the results are as follows: image 3 shown). Figure 4 It is shown that only UGT1A1 can catalyze the reaction, and the reaction rate is much higher than that of other ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com