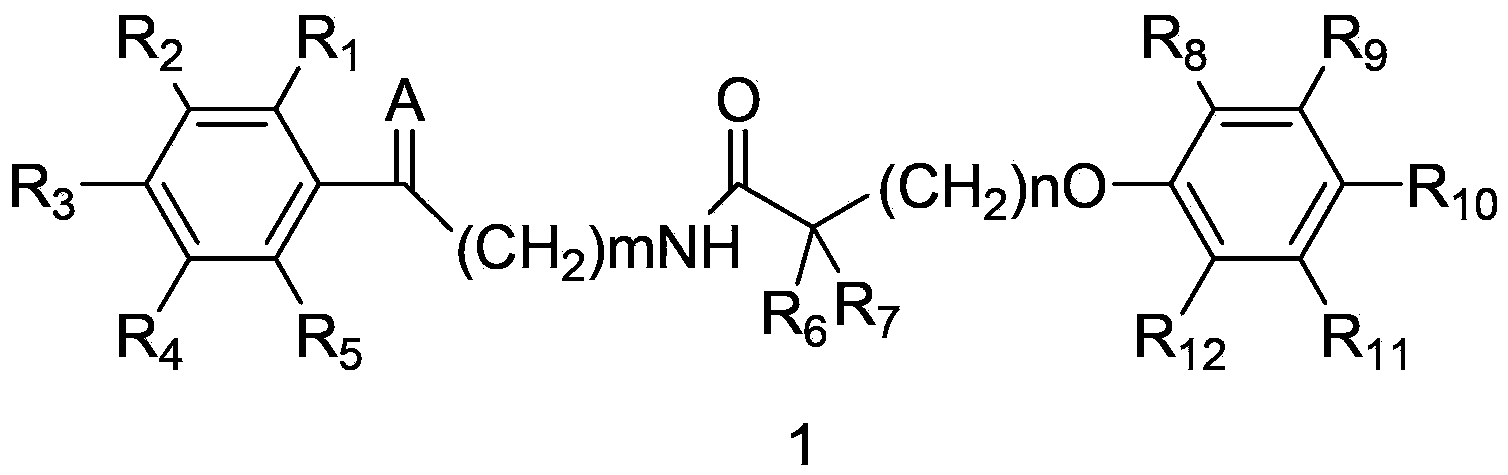
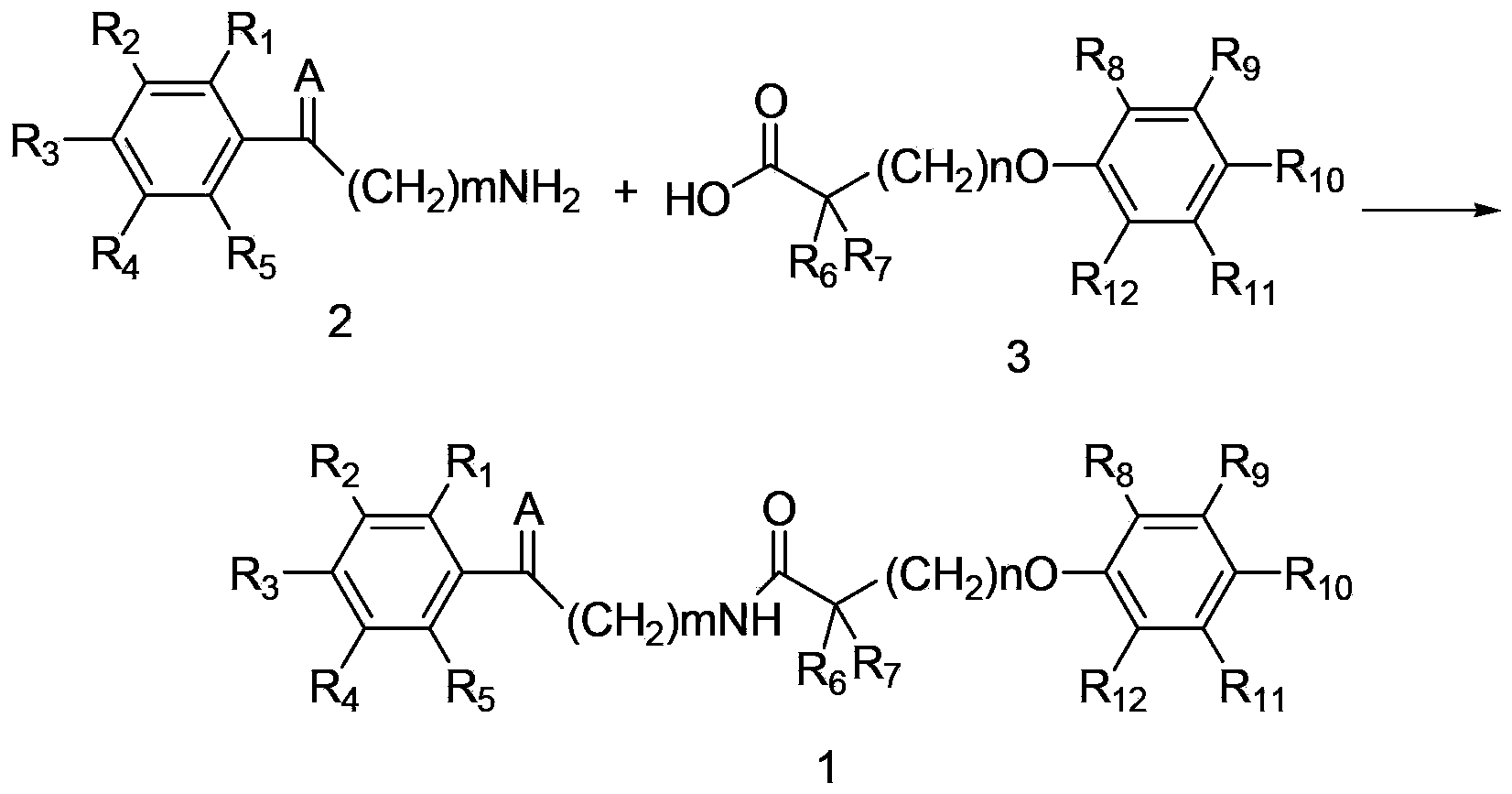
Amide compound and preparation method, medicinal composition and application thereof
A technology of amide compounds and drugs, which is applied in the field of amide compounds and can solve the problems of poor effect and large side effects of blood lipid-lowering drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0177] Example 1: Preparation of amide compound 1-1
[0178] 2-(4-(4-chlorobenzoyl)phenoxy)-2-methylpropionyl chloride 1g, 2-aminoacetophenone 0.4g, triethylamine 0.3g, dichloromethane 60mL placed in 100mL In a single-neck flask, react for 4 hours at room temperature, evaporate the solvent, and obtain the target amide compound 1-11.09g by column chromatography with a yield of 84%. MS (ESI): 436[M+H + ].
[0179] The amide compound 1-2 and the amide compound 1-3 can be prepared by a method similar to that described in Example 1 using corresponding raw materials. Amide compound 1-2: MS (ESI): 368[M+H + ]. Amide compounds 1-3: MS (ESI): 479[M+H + ].
Embodiment 2
[0180] Example 2: Preparation of amide compound 1-1
[0181] 2-methyl-2-(4-(4-chlorobenzoyl)phenoxy)propionic acid 1g, dicyclohexylcarbodiimide 0.57g, 2-aminoacetophenone 0.33g, dichloromethane 60mL Placed in a 100mL single-necked flask, reacted at room temperature for 4 hours, evaporated the solvent, and column chromatography gave the target amide compound 1-11.12g with a yield of 82%.
Embodiment 3
[0182] Example 3: Preparation of amide compounds 1-4
[0183] 5-(2,5-dimethylphenoxy)-2,2-dimethylvaleryl chloride 1g, 2-amino-(4-bromophenyl)-ethanone 0.79g, triethylamine 0.38g, Put 60 mL of dichloromethane in a 100 mL single-necked flask, react for 4 hours at room temperature, evaporate the solvent, and obtain 1-41.35 g of the target amide compound by column chromatography with a yield of 81%. MS (ESI): 446[M+H + ].
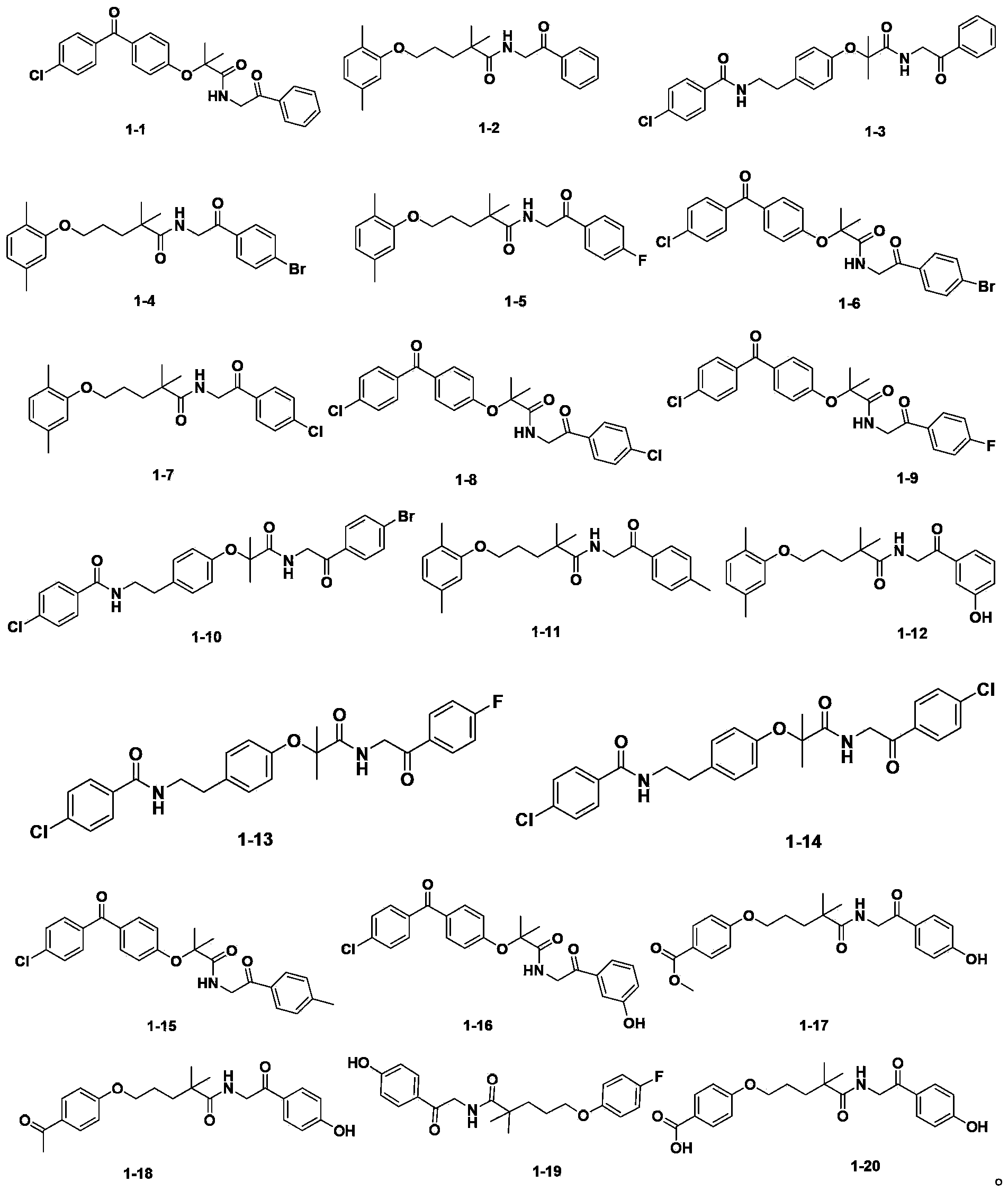
[0184] Amide compounds 1-5 to amide compounds 1-16 can be prepared by using the similar method described in Example 3 with corresponding raw materials.
[0185] Amide compounds 1-5: Yield 83%, MS (ESI): 386[M+H + ].
[0186] Amide compound 1-6: yield 87%, MS (ESI): 514[M+H + ].
[0187] Amide compounds 1-7: Yield 79%, MS (ESI): 402[M+H + ].
[0188] Amide compounds 1-8: Yield 76%, MS (ESI): 470[M+H + ].
[0189] Amide compounds 1-9: Yield 82%, MS (ESI): 454[M+H + ].
[0190] Amide compounds 1-10: 85% yield, MS (ESI): 557[M+H + ].
[0191] Amide compounds 1-11: Yield 77%, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size (mesh) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com