In vitro biliary excretion assay
一种胆汁排泄、胆汁的技术,应用在体外胆汁排泄测定法领域,能够解决低精确性等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0137] Example 1: Hepatocyte-mesenchymal cell co-culture
[0138] Cryopreserved human hepatocytes were removed from liquid nitrogen and thawed. After thawing, cells were resuspended in culture medium and cell number and cell viability were determined using trypan blue exclusion. stromal cells in CO 2 Passage in the incubator until used for experimental plating. On the day of plating, cells were detached from the plate, washed, and resuspended in culture medium. Cell number and viability were determined using trypan blue exclusion.
[0139] Hepatocytes and stromal cells were seeded into collagen-coated 96-well plates at a density of 30,000 hepatocytes per well. Stromal cells were subjected to growth arrest prior to seeding. Cultures were maintained for 7 days before starting any biliary excretion assays. Cells were stained with CDFDA on day 7 to ensure tubule formation before starting the experiment.
Embodiment 2
[0140] Example 2: Excretion of test compounds into bile canaliculi in hepatocyte-stromal cell co-cultures
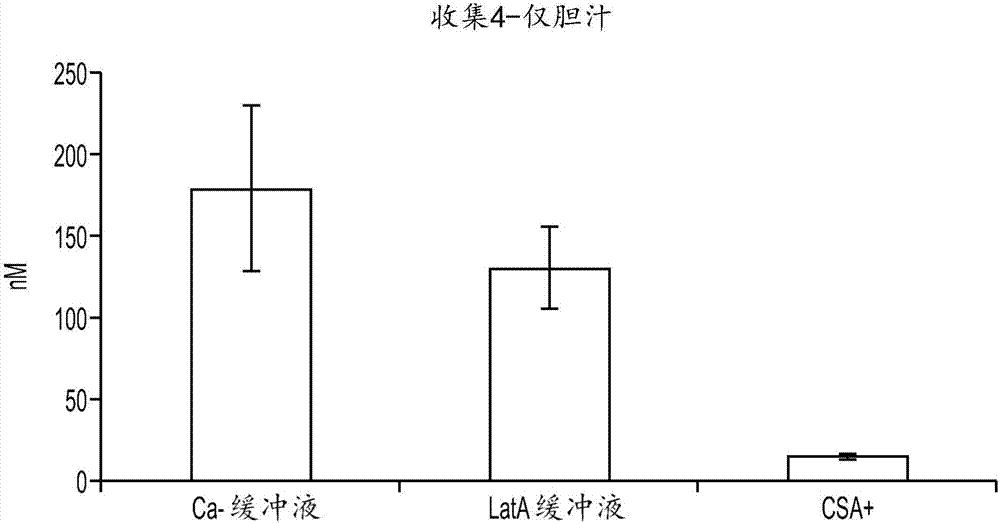
[0141]In this experiment, the presence of bile canaliculi in hepatocyte-stromal cell cultures prepared according to Example 1 was assessed. Co-cultures were incubated with 5 μM 5-(and-6)-carboxy-2',7'-dichloro-fluorescein diacetate (CDFDA) for 20 min. CDFDA uptake by hepatocytes is hydrolyzed by hepatocytes to fluorescent 5-(and-6)-carboxy-2',7'-dichloro-fluorescein (CDA), which is then secreted into tubules in this fluorescent form by the Mrp2 transporter middle. Co-cultures included several tubules that were readily apparent under the microscope (data not shown). Cyclosporin A (CSA) is a known inhibitor of the MrP2 transporter. In subsequent experiments, co-cultures were incubated with CDFDA for 20 min in the presence of 50 μM CSA. After incubation with CDFDA in the presence of CSA, no fluorescent tubules were visible (data not shown). This result demonstrates the...
Embodiment 3
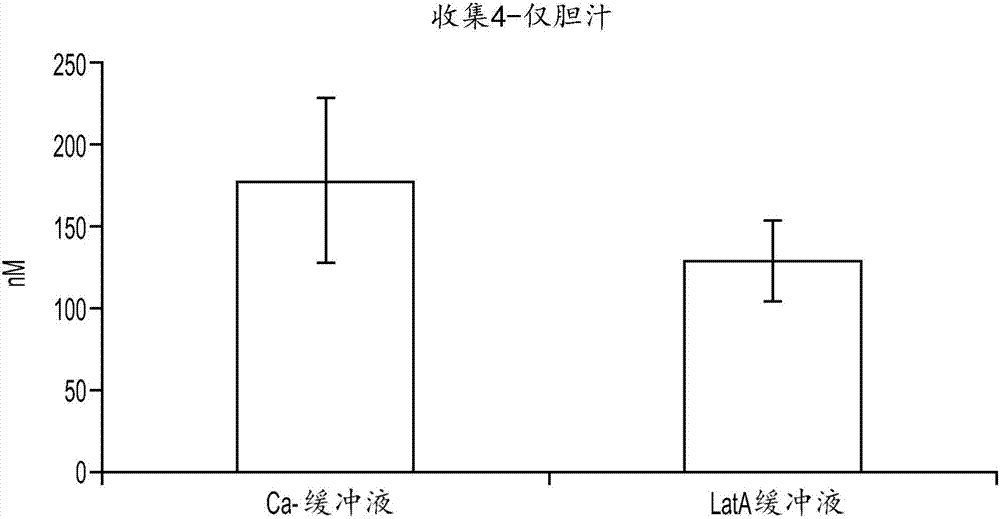
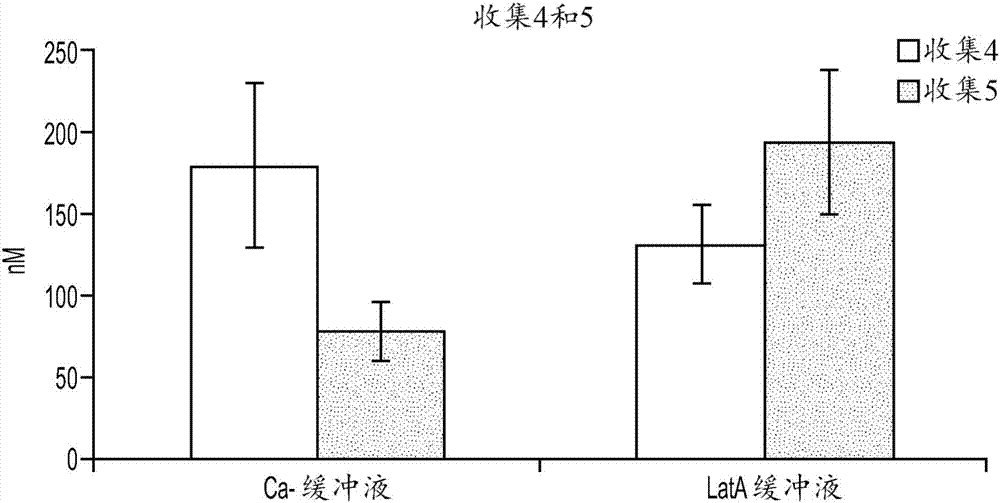
[0142] Example 3: Disruption of tubules
[0143] Assessing the excretion of chemical entities into bile in hepatocyte-stromal cell co-cultures requires a method to distinguish chemical entities present in the tubules of the co-culture from those that may be present in the cytoplasm of cells in the co-culture. Two methods of tubule disruption were tested. In the first method, cultures are exposed to a calcium (Ca-)-free buffer. The lack of calcium inhibits the cells' ability to form tight junctions, which allow bile present in the tubules to escape. First, cells were washed twice in HBSS buffer with Ca+. Cells were then incubated with 5 μM CDFDA in Ca-free HBSS buffer for 20 minutes. Then, cells were washed twice in Ca-free HBSS buffer. No CDF staining was evident as accumulated CDF escaped the tubules after exposure to Ca-buffer (data not shown).
[0144] In the second method, cultures were exposed to a buffer containing Lachunkulin A (LatA). LatA functions to disrupt mi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com