Derivatives of astilbin and preparation method thereof
A technology for astilbin and its derivatives, which is applied in the field of astilbin derivatives and their preparation, and can solve problems such as limiting clinical applications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
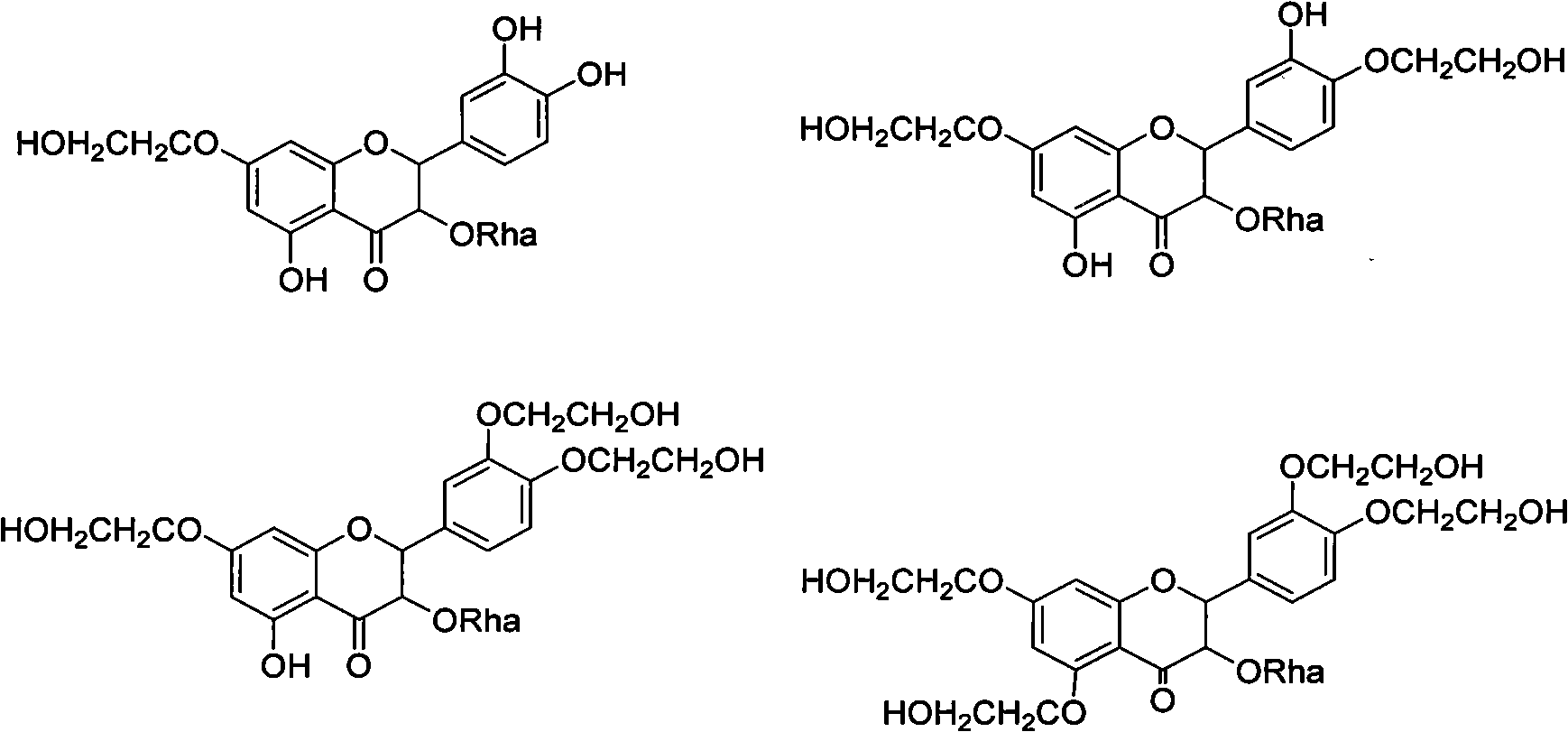
[0047] 1. Prepare the compound of the following formula:
[0048]
[0049] Dissolve astilbin in NaOH solution, pass through ethylene oxide, react at 70°C-75°C for 3 hours, let cool, add water and place overnight to obtain crude product, and obtain pure product through repeated column chromatography.
[0050] Chemical Formula: C23H26O12, Exact Mass: 494.14, Molecular Weight: 494.45, m / z: 494.14 (100.0%), 495.15 (25.6%), 496.15 (5.6%), Elemental Analysis: C, 55.87; H, 5.30; O, 38.83, Boiling Point: 1573.51[K], Melting Point: 1171.21[K], Critical Temp: 1343.6[K], Critical Press: 30.83[Bar], Critical Vol: 1193.5[cm3 / mol], Gibbs Energy: -1133.08[ kJ / mol], Log P: -0.56, MR: 118.94 [cm3 / mol], Henry's Law: 31.6, Heat of Form: -1855.66 [kJ / mol], tPSA: 245.3, CLogP: -0.0688307, CMR: 11.6666.
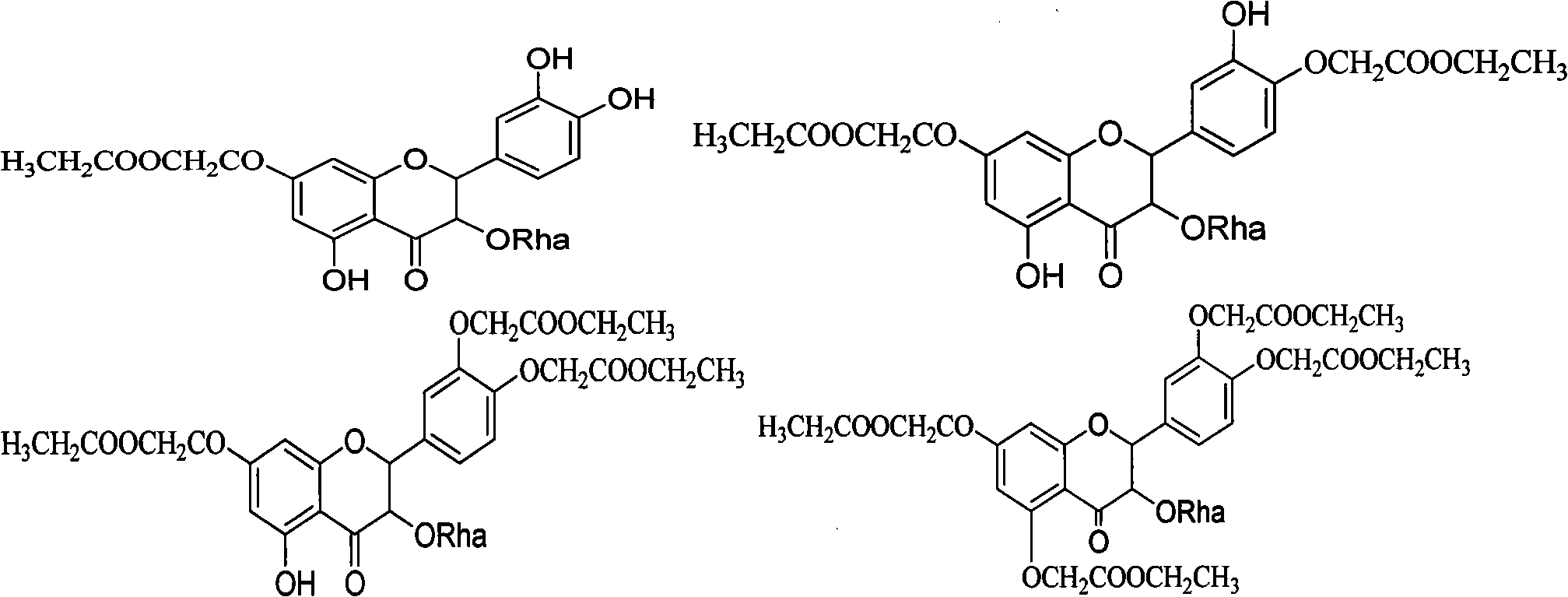
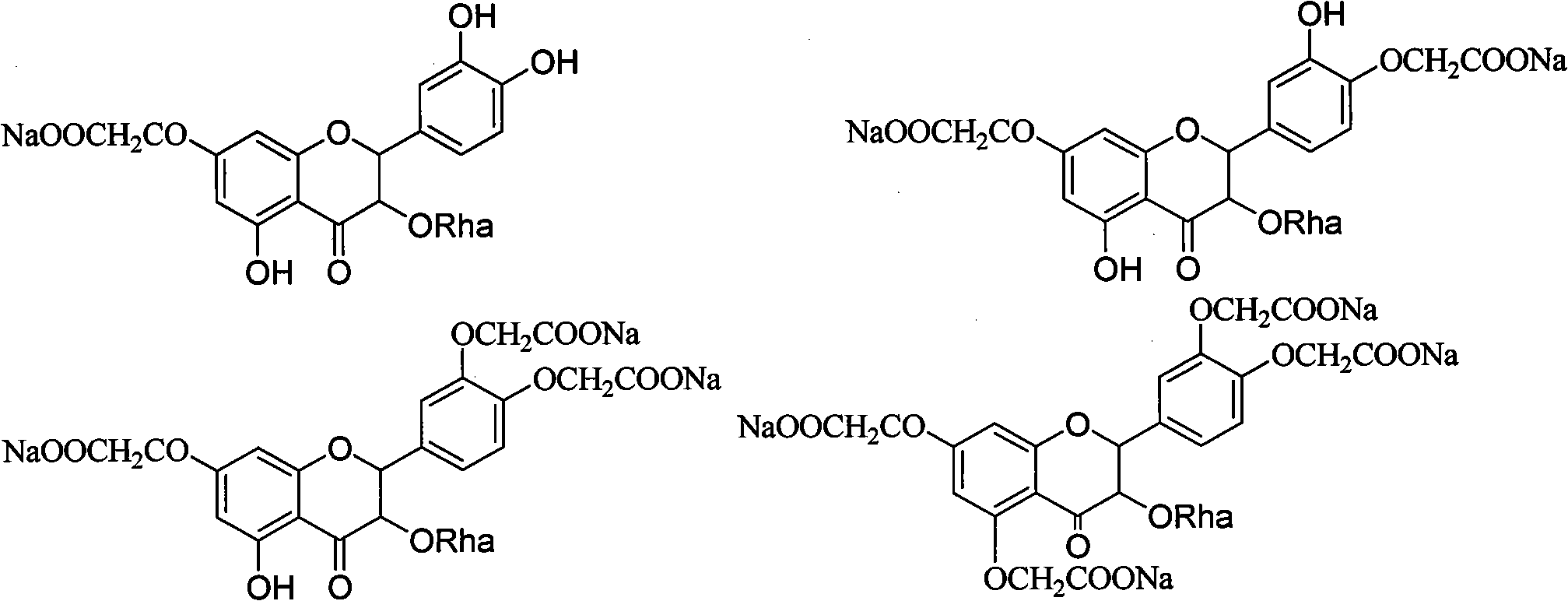
[0051] 2. Prepare the compound of the following formula:
[0052]
[0053] Add astilbin, DMF and anhydrous potassium carbonate into the three-necked flask, add ethyl chloroacetate dropwise...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com