Quality inspection method of Wujiaqi oral liquid (Chinese patent medicine prepared from acanthopanax root and radix astragali)
A technique for Wujiaqi oral liquid and Jiaqi oral liquid is applied in the field of quality inspection of Wujiaqi oral liquid, which can solve problems such as unfavorable quality and no quality standards, and achieve the effects of safety in clinical use and good accuracy.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Embodiment 1 prepares Acanthopanax oral liquid
[0053] Wujiaqi Oral Liquid: The prescription includes 800g of Astragalus and 200g of Acanthopanax.
[0054] Preparation method: crush the above two traditional Chinese medicines into the coarsest powder, add 8 times the amount of water, soak for 1 hour, decoct and reflux for extraction for 2 hours, filter, add water to the filter residue and extract twice, add 8 times the amount of water each time and reflux for 2 hours, Filter several times, combine the filtrates, centrifuge while hot (50-60°C), concentrate the supernatant to a relative density of 1.05-1.08 (≤70°C), refrigerate for 12-24 hours, centrifuge, and concentrate the supernatant to a relative density of 1.15 under reduced pressure ~1.23 (≤70°C), then refrigerated for 12~24h, centrifuged, added purified water to the supernatant to 1000ml, stirred well, filtered, and packaged to obtain.
Embodiment 2 5
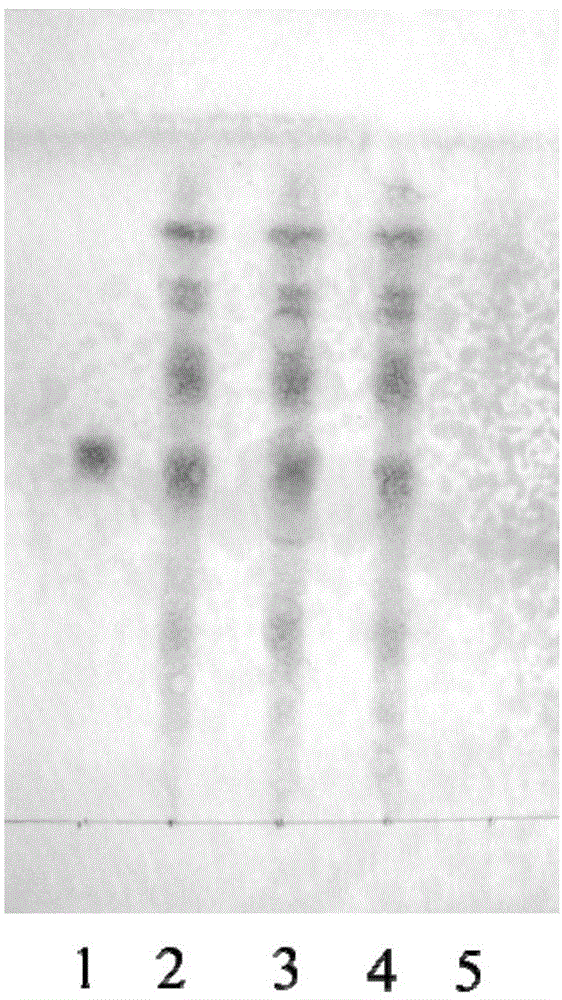
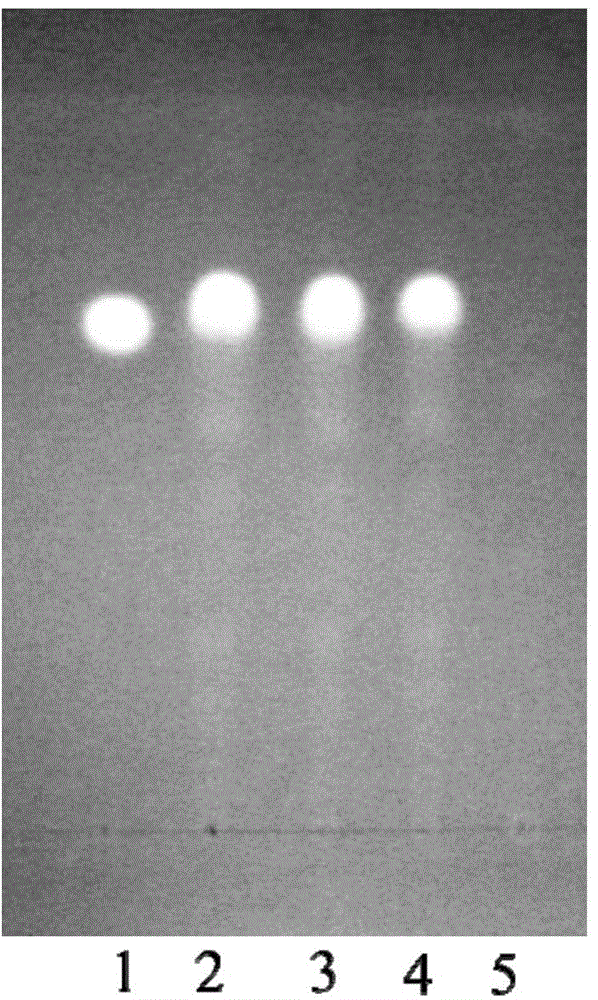
[0055] Example 2 Examination of Specificity of Identification Method of Wujiaqi Oral Liquid
[0056] (1) Reagents and samples
[0057] Control samples: Astragaloside IV, batch number: 110781-200613, purchased from China National Institutes for Food and Drug Control;
[0058] Acanthopanax oral liquid: prepared in Example 1.
[0059] Negative control samples: Prepare negative control samples for each identification item according to the method in Example 1.
[0060] (2) Identification of Radix Astragali
[0061] Take 3ml of the sample prepared in Example 1, add 27ml of water, shake and extract 2 times with water-saturated n-butanol, 20ml each time, combine the n-butanol solution, wash 2 times with water, 20ml each time, discard the water solution, and n-butanol Evaporate the alcohol solution to dryness, add 1ml of methanol to the residue to dissolve it, and use it as the sample solution to be tested; take another control sample of astragaloside IV, add methanol to make a solu...
Embodiment 3
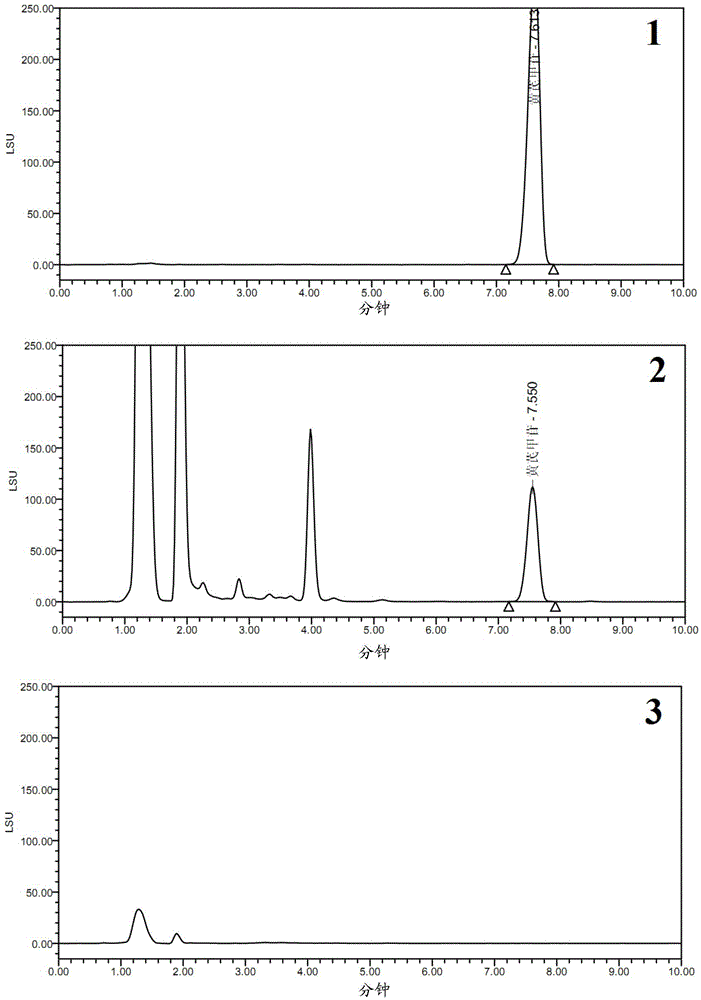
[0066] Example 3 Astragaloside IV content detection methodological investigation
[0067] (1) Instruments and reagents, samples
[0068] instrument:
[0069] High performance liquid chromatography, type e2695, Waters Corporation, USA; evaporative light scattering detector, type 2424, Waters Corporation, USA; electronic analytical balance, type AB135-S, Mettler Toledo Corporation.
[0070] Reagents: Acetonitrile is chromatographically pure; water is double distilled water; other reagents are analytically pure.
[0071] Control sample: Astragaloside IV, batch number: 110781-200613, purchased from China National Institutes for Food and Drug Control.
[0072] Acanthopanax oral liquid: prepared in Example 1.
[0073] (2) Chromatographic conditions
[0074] The chromatographic column is a Kromasil100-5C18 column (150×4.6mm, 5μm); the mobile phase is acetonitrile-water (35:65); detection by an evaporative light scattering detector, the temperature of the drift tube is 60°C, the t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com