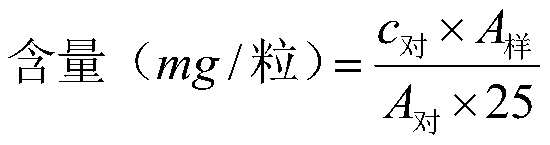Quality control method of capsule preparation for treating gastric ulcer
A quality control method and gastric ulcer technology, applied in the direction of measuring devices, instruments, scientific instruments, etc., can solve problems such as difficult to effectively control quality, and not provide effective chemical components for quality control, so as to achieve scientific and reasonable quality control methods and ensure clinical Good curative effect and reproducible effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] The quality control method of Liangjiang Weiyang Capsules for treating gastric ulcer is mainly content determination, and the contents of 7 components in Liangjiang Weiyang Capsules are determined simultaneously by HPLC method;
[0057] Chromatographic conditions and system suitability test: use octadecylsilane bonded silica gel as filler; methanol (A)-0.1% formic acid water (B) as mobile phase; gradient elution (0-8min, 10%-21% A; 8~14min, 21%A; 14~20min, 21%~29%A; 20~29min, 29%A; 29~35min, 29%~55%A; 35~42min, 55%A~73% A; 42~60min, 73%~100%A); the detection wavelength is 267nm (evodiamine) and 344nm (scopoletin, dehydroevodiamine, scopoletin, isoxapidine, evodiamine, evodiamine base), the number of theoretical plates should not be less than 5000 based on the evodiamine peak;
[0058] Preparation of reference solution: Accurately weigh 12.91 mg of scopoletin, 8.04 mg of dehydroevodiamine, 6.51 mg of scopoletin, 9.99 mg of isoxazinidine, 5.75 mg of evodiamine, 6.90 mg o...
Embodiment 2
[0066] The quality control method of the capsule preparation for the treatment of gastric ulcer, using the HPLC method to determine the content of scopoletin, dehydroevodiamine, scopoletin, isoxazinidine, evodiamine, evodiamine, and evodiamine in Liangjiang Weiyang capsules content;
[0067] The method comprises the following steps: taking the test substance and the reference substance of Galangal Weiyang Capsules for detection, and the chromatographic conditions for the detection are: chromatographic column: octadecylsilane bonded silica gel; mobile phase: methanol (A)-0.1% formic acid aqueous solution (B); flow rate: 1mL / min; column temperature: 30°C; detection wavelength: 267 and 344nm; elution gradient: 0-8min, 10%-21% (A); 8-14min, 21% (A) ;14-20min, 21%-29%(A); 20-29min, 29%(A); 29-35min, 29%-55%(A); 35-42min, 55%-73%(A); 42-60min, 73%-100% (A).
Embodiment 3
[0069] The quality control method of the capsule preparation for the treatment of gastric ulcer specifically comprises the following steps:
[0070] (1) Preparation of the reference solution: Accurately weigh 12.91 mg of scopoletin, 8.04 mg of dehydroevodiamine, 6.51 mg of scopoletin, 9.99 mg of isopyridine, 5.75 mg of evodiamine, 6.90 mg of evodiamine, Evodicarpine 5.00mg, with methanol as solvent, prepared to concentrations of 0.5164mg / mL, 0.2251mg / mL, 0.1302mg / mL, 0.2398mg / mL, 0.1093mg / mL, 0.1794mg / mL, 0.0500mg / mL Mix the reference substance solution, shake well, and store in a 4°C refrigerator for later use;
[0071] (2) Preparation of the test solution: Weigh 0.5 g of the contents of the same batch of capsules, put them into a flat-bottomed flask, add 25 mL of methanol with a volume concentration of 90%, weigh the weight, heat and reflux for 0.5 h, cool to room temperature, and use The volume concentration is 90% methanol to make up the weight, shake well, and filter to ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com