Method for detection, identification and content determination of folia eriobotryae or drug containing folia eriobotryae raw material
A detection method, loquat leaf technology, applied in the field of drug quality detection, to achieve the effect of saving detection costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
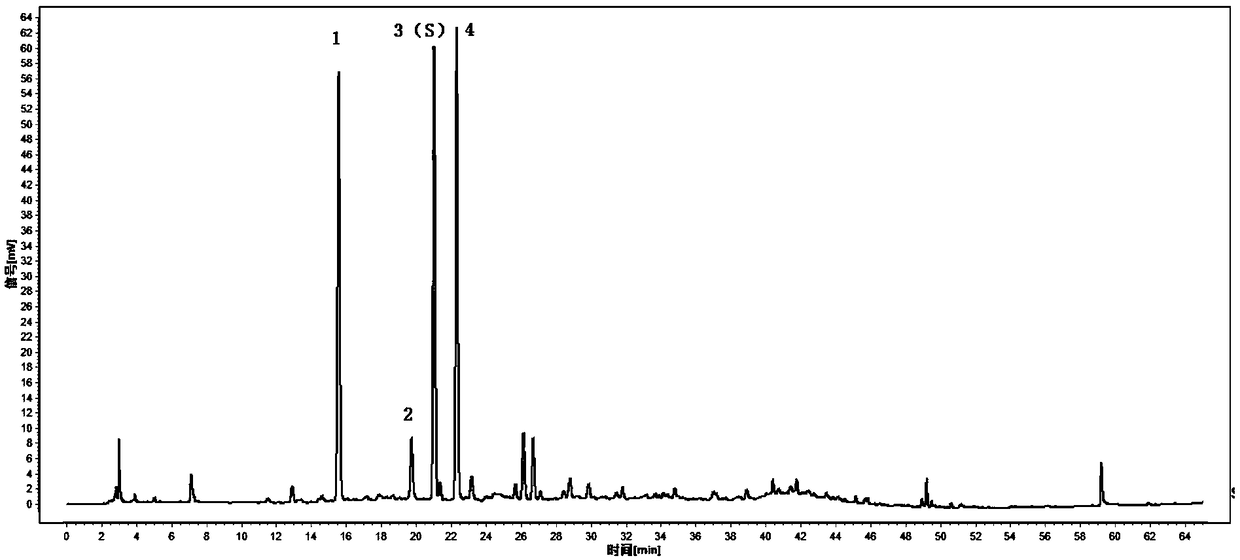
[0058] Embodiment 1 HPLC characteristic collection of illustrative plates of the present invention
[0059] 【Characteristic spectrum】Determined according to high performance liquid chromatography ("Chinese Pharmacopoeia" 2015 edition four general rules 0512).
[0060]Chromatographic conditions and system suitability test: Octadecylsilane bonded silica gel is used as filler (column length is 250mm, inner diameter is 4.6mm, particle size is 5μm); 0.4% phosphoric acid solution is used as mobile phase A, and acetonitrile is used as mobile phase B. Perform gradient elution as specified in the table below; the flow rate is 1.0 mL per minute; the column temperature is 35°C; the detection wavelength is 300 nm. The number of theoretical plates should not be less than 5000 based on the chlorogenic acid peak.
[0061]
[0062] Preparation of reference solution: Take appropriate amount of reference substances of chlorogenic acid, neochlorogenic acid, and cryptochlorogenic acid, add 50...
Embodiment 2
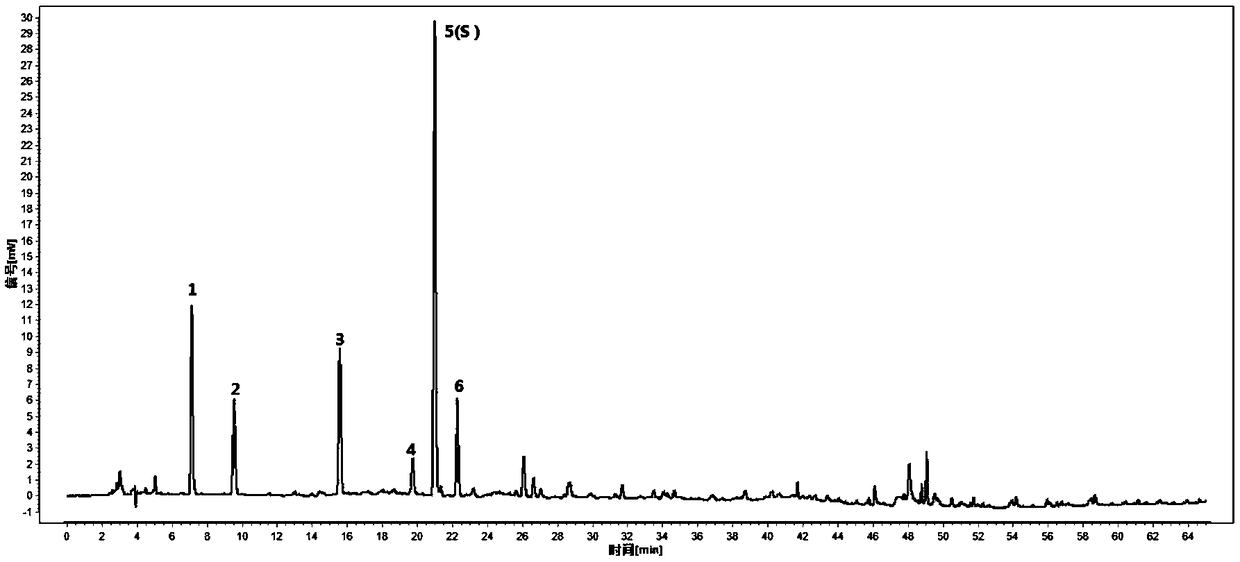
[0068] The conditional screening test of embodiment 2 characteristic graph of the present invention
[0069] 1. Experimental instruments and materials
[0070] High performance liquid chromatography: Agilent 1260 high performance liquid chromatography, Shimadzu LC-20AD high performance liquid chromatography, Waters 2695-2996 high performance liquid chromatography;
[0071] Electronic balance: ME204E / 02, MS205DU, XP26 (Mettler-Toledo Instrument Co., Ltd.);
[0072] Ultrapure water machine: cell type 1810A (Shanghai Mole Scientific Instrument Co., Ltd.);
[0073] Ultrasonic cleaner: KQ5200DB type (600W, 40KHz; Kunshan Ultrasonic Instrument Co., Ltd.);
[0074] Chromatographic column: Agilent5HC-C18 250×4.6mm 5μm, Diamonsil C18 250×4.6mm 5μm, KromasilC18 5um 4.6×250mm.
[0075] Acetonitrile, methanol, and phosphoric acid are chromatographically pure, water is ultrapure water, and other reagents are analytically pure.
[0076] Chlorogenic acid (National Institute for Food and ...
Embodiment 3
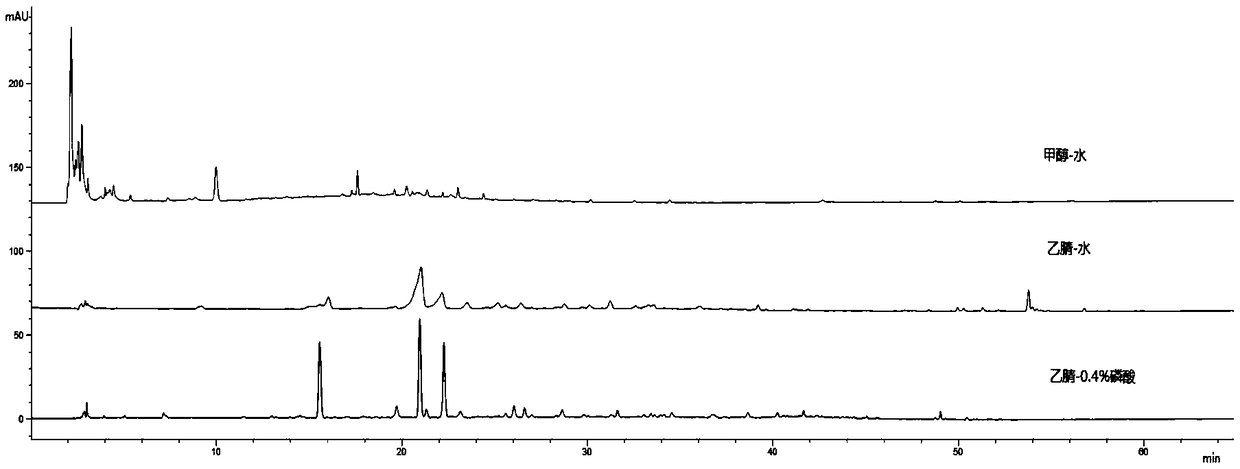
[0177] Embodiment 3 Assay method of the present invention
[0178] 1 Instruments and reagents
[0179] High performance liquid chromatography: Agilent 1260 high performance liquid chromatography, Shimadzu LC-20AD high performance liquid chromatography, Waters2695-2996 high performance liquid chromatography;
[0180] Electronic balance: ME204E / 02, MS205DU, XP26 (Mettler-Toledo Instrument Co., Ltd.);
[0181] Ultrapure water machine: cell type 1810A (Shanghai Mole Scientific Instrument Co., Ltd.);
[0182] Ultrasonic cleaner: KQ5200DB type (600W, 40KHz; Kunshan Ultrasonic Instrument Co., Ltd.);
[0183] Chromatographic column: Agilent 5TC-C18(2) 250×4.6mm, phenomenonexLuna 5u C18(2) 100A250×4.60mm 5micron, kromasilC18 5um4.6×250mm.
[0184]Chlorogenic acid (National Institute for Food and Drug Control, batch number: 110753-201415, with a content of 96.2%).
[0185] Acetonitrile and phosphoric acid are chromatographically pure, water is ultrapure water, and other reagents are...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com