Pharmaceutical composition useful for treating chronic myeloid leukemia
a technology of myeloid leukemia and pharmaceutical composition, which is applied in the direction of drug compositions, peptide sources, peptide/protein ingredients, etc., can solve the problems of poor prognosis of cml
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
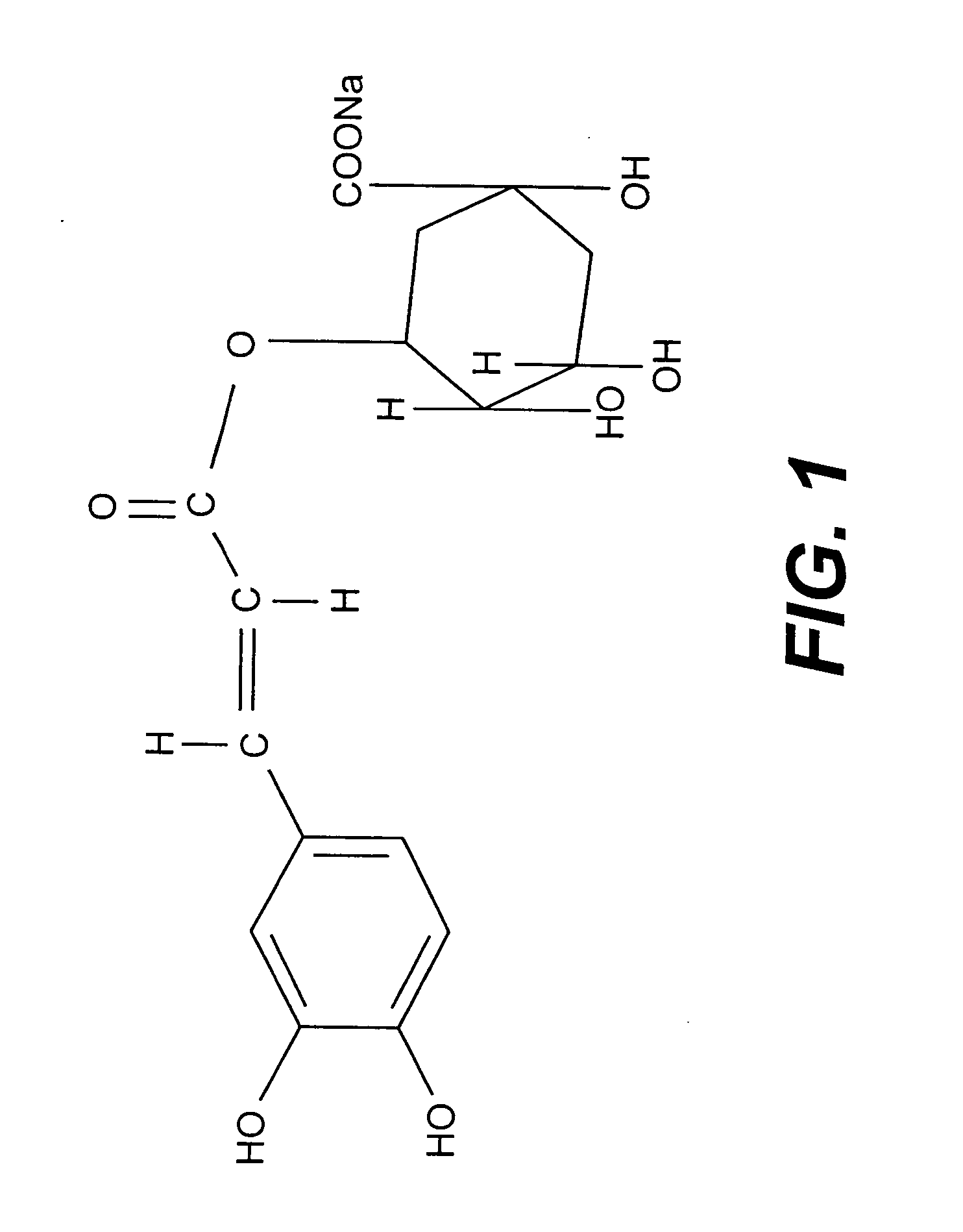
[0057] Preparation of Sodium Chlorogenate:
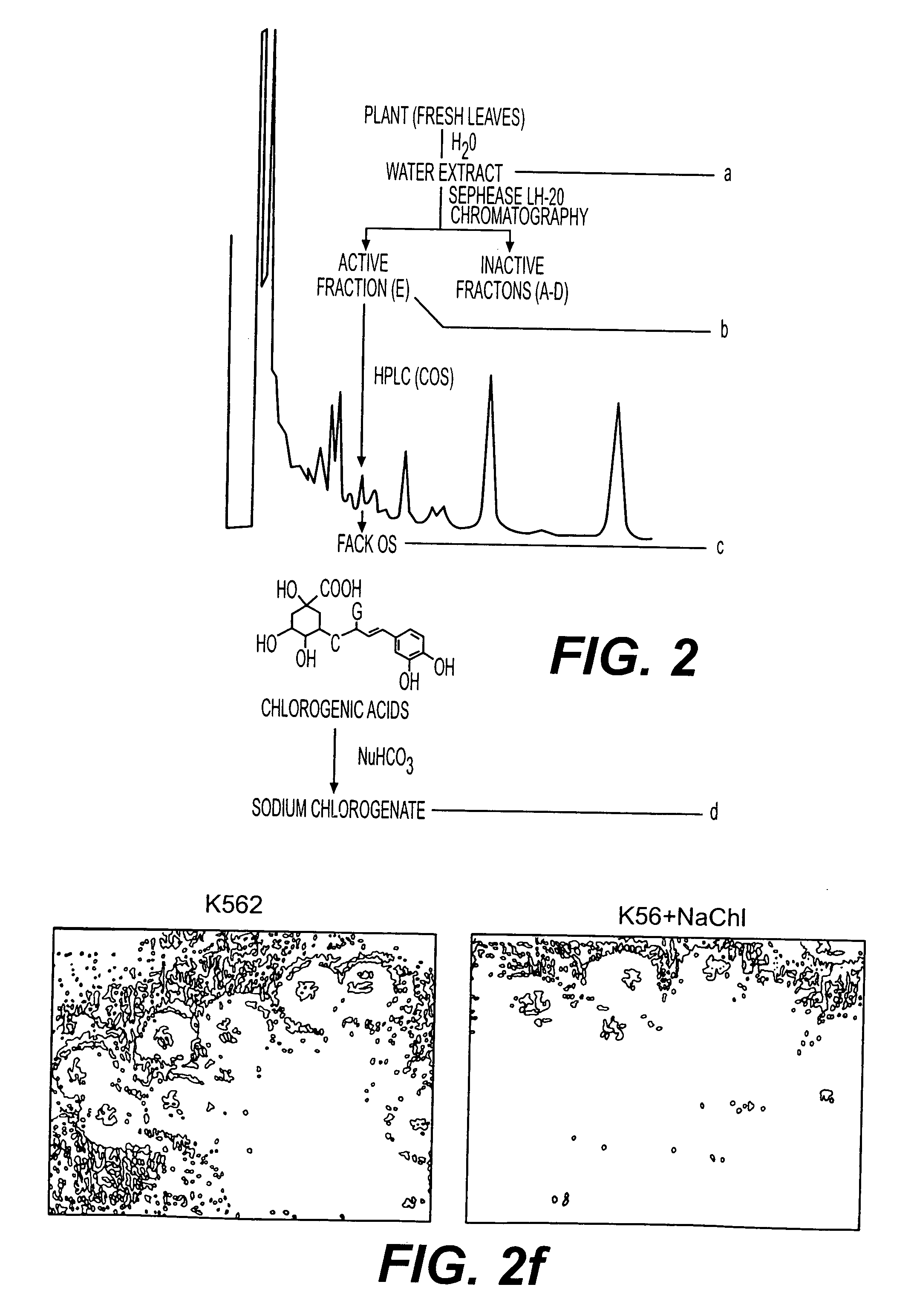
[0058] 4.7 kg of Piper betel leaves freshly collected, washed with distilled water and then cut into small pieces. Small pieces of leaves were gathered together and mixed with 1.0 litre of distilled water and thoroughly homogenized in a mixture blender. The homogenate was passed through a fine cheesecloth to filter out the large particles and the filtrate was collected. The process was repeated 2-3 times to have maximum yield. The combined filtrate was then centrifuged, the aliquot, a clear solution, was collected and lyophilised to a semi-solid mass, which was about 110 gm. Collected material was examined for biological activity i.e. destruction of CML cells. On observing its positive activity, purification was initiated. 10 gm of above-mentioned material was loaded on Sephadex LH-20 column and chromatographed with water, water-methanol (1:1) and methanol as eluent. Three different fractions thus obtained from three different solvent system...
example 2
[0060] The Chlorogenic acid is available in the market in the pure form. The Chlorogenic acid (1 gm) was hand shaken with sodium hydrogen carbonate (024 g in 5 ml of water) solution. The solution was lyophilised to pure sodium chlogogenate (1.12 gm) and was tested for biological activity. Sodium chlorogenate prepared from chlorogenic acid which was either isolated from Piper betel or obtain commercially have similar structure and activity.
example 3
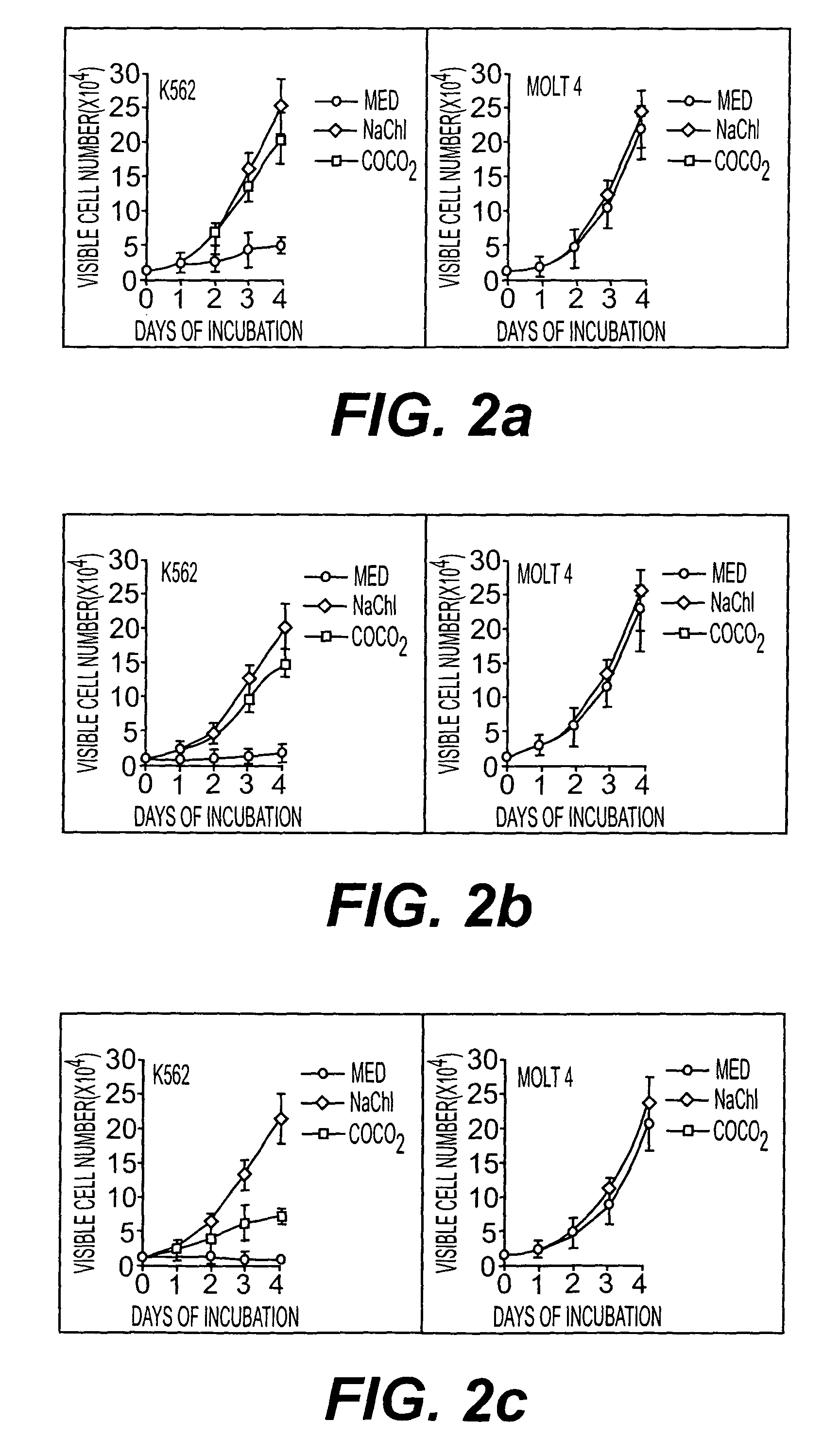
[0061] Culture of Bcr-Abl positive CML cell line (K562), peripheral blood cells of CML patients, Bcr-Abl-negative ALL cell line (Molt-4) and peripheral blood cells of CML patients. Cell count assays were performed by plating cells in the presence of regular growth medium with or without indicated amount of extract, fraction, purified compound and its sodium salt. Each day, viable cells were counted as assessed by exclusion of trypan blue.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Time | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
| Dimensionless property | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com