Phlegm-heat clearing injection fingerprint spectrum establishment method and fingerprint spectrum thereof
A technology of fingerprint spectrum and establishment method, which is applied to the establishment method of Tanreqing injection fingerprint spectrum and the field of fingerprint spectrum, can solve the problems of difficult to achieve effective separation, complex chemical composition and the like, and achieves stable results, good reproducibility, Avoid one-sided effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
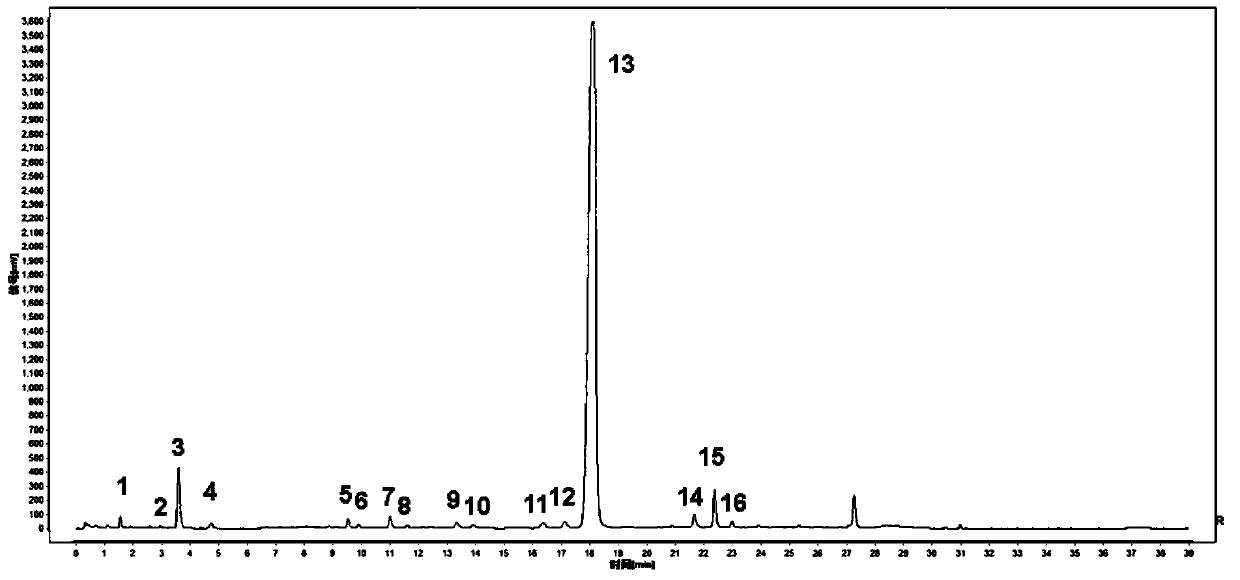
[0055] The establishment method of Tanreqing injection fingerprint of embodiment 1 comprises the following steps:
[0056] 1. Instruments and reagents
[0057] Agilent 1290 high performance liquid chromatograph (Agilent Corporation, USA).
[0058] Reagents: Acetonitrile and methanol are chromatographically pure, and other reagents are analytically pure.
[0059] Caffeic acid, chlorogenic acid, baicalin, scutellarin, and wogonin were all purchased from China Institute for the Control of Pharmaceutical and Biological Products, neochlorogenic acid, cryptochlorogenic acid, 3,4-dicaffeoylquinic acid, 3, 5-dicaffeoylquinic acid, 4,5-dicaffeoylquinic acid, and forsythiaside E were all purchased from Shanghai Tongtian Biotechnology Co., Ltd. Chrysin-7-O-glucuronide , Melaleucain-7-O-glucuronide were purchased from Jiangsu Yongjian Pharmaceutical Technology Co., Ltd.
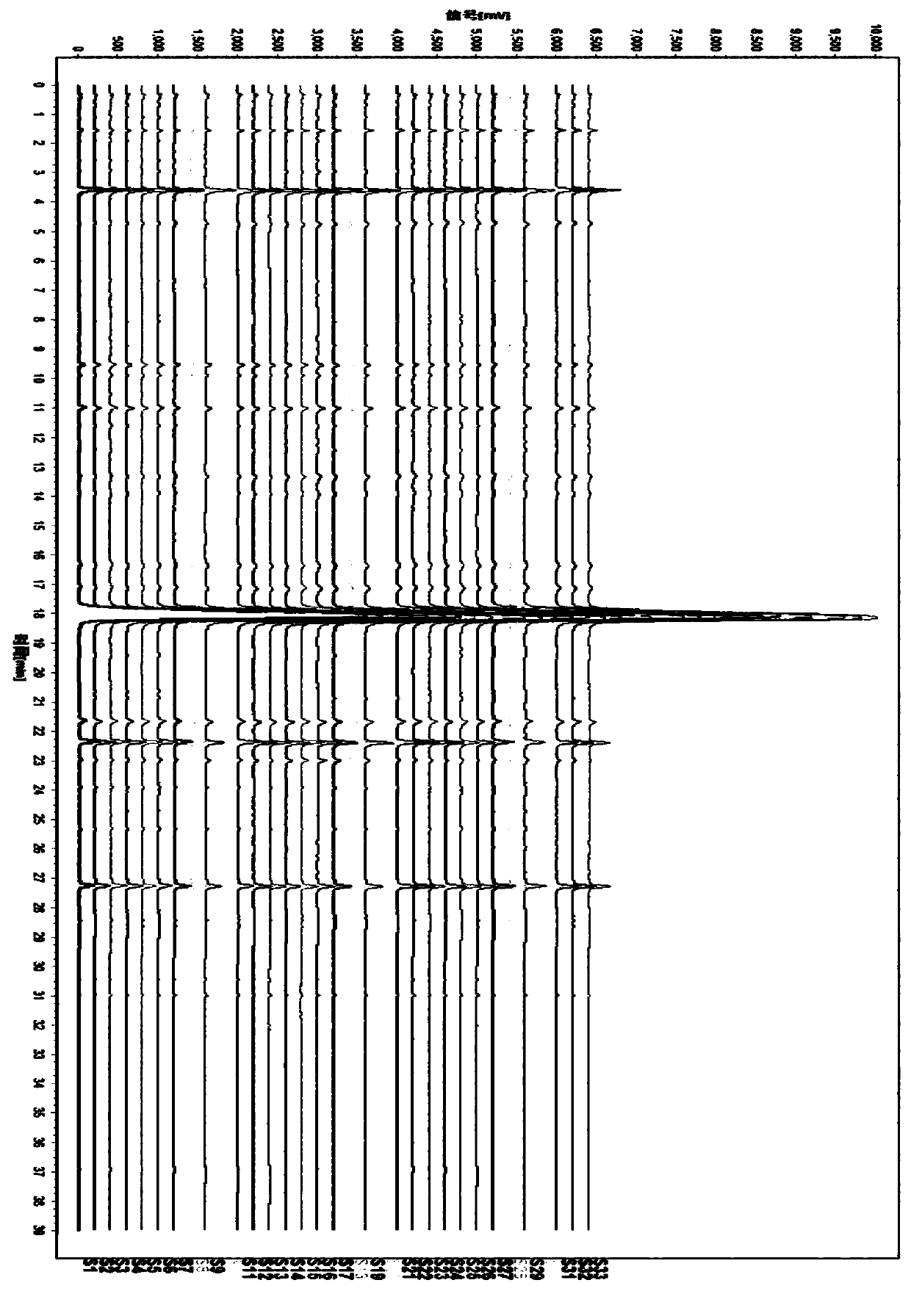
[0060] Test drug: a total of 33 batches of Tanreqing Injection, the sample information is shown in Table 1:
[006...
Embodiment 2
[0075] 2. Chromatographic conditions: with octadecylsilane bonded silica gel as filler; with acetonitrile as mobile phase A, with 0.1% formic acid solution as mobile phase B, carry out gradient elution according to the provisions in Table 3 (after the peak is completed Wash the chromatographic column with a high proportion of acetonitrile), the flow rate is 0.8ml / min, the column temperature is 30°C, and the detection wavelength is 280nm.
[0076] Table 3 Gradient elution program
[0077] time (minutes) Mobile phase A(%) Mobile phase B(%) 0~5 95 5 5~7 95→89 5→11 7~18 89→86 11→14 18~27 86→71 14→29 27~31 71→20 29→80
Embodiment 3
[0079] 2. Chromatographic conditions: with octadecylsilane bonded silica gel as filler; with acetonitrile as mobile phase A, with 0.1% formic acid solution as mobile phase B, carry out gradient elution according to the provisions in Table 4 (after the peak is completed Wash the chromatographic column with a high proportion of acetonitrile), the flow rate is 0.8ml / min, the column temperature is 30°C, and the detection wavelength is 280nm.
[0080] Table 4 Gradient elution program
[0081] time (minutes) Mobile phase A(%) Mobile phase B(%) 0~6 95 5 6~9 95→90 5→10 9~20 90→83 10→17 20~28 83→75 17→25 28~31 75→20 25→80
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com