Quality monitoring method for quantitative fingerprint spectrum of Shuanghuanglian oral liquid
A technology of Shuanghuanglian oral liquid and fingerprint spectrum, which is applied in the field of compound quality analysis, can solve the problems of difficult to truly reflect the content of caffeoylquinic acids, cumbersome and time-consuming methods, and lack of overall fingerprint control, and achieve a comprehensive analysis method. and the effect of the difference analysis strategy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0035] 1. Analysis conditions:
[0036] Instrument: Alliance e2695-2998
[0037] Chromatographic column: Tnature C18 (Acchrom-Tech, 4.6×250mm, 5μm)
[0038] Flow rate: 1mL / min
[0039] Column temperature: 40°C
[0040] Injection volume: 10μL
[0041] Wavelength collection range: 190nm-600nm
[0042] Extraction wavelength: 235nm, 327nm
[0043] Mobile phase: A.0.1% phosphoric acid / acetonitrile (v / v) B.0.1% phosphoric acid / water (v / v)
[0044] gradient:
[0045]
[0046]
[0047] 2. Accurately weigh the reference substance of chlorogenic acid, add water to make a stock solution containing 1000 μg of chlorogenic acid per 1 mL; take the reference substance of forsythin, add 50% methanol / water to make 1000μg stock solution of baicalin; accurately weigh the reference substance of baicalin, add 10% of the total volume of the stock solution with N,N-dimethylformamide to dissolve completely, then add methanol to make a stock solution containing 1000μg of baicalin per 1mL li...
Embodiment 2
[0051] method of execution
[0052] 1. Analysis conditions:
[0053] Instrument: Alliance e2695-2998
[0054] Chromatographic column: Tnature C18 (Acchrom-Tech, 4.6×250mm, 5μm)
[0055] Flow rate: 1.2mL / min
[0056] Column temperature: 40°C
[0057] Injection volume: 10μL
[0058] Wavelength collection range: 190nm-600nm
[0059] Extraction wavelength: 235nm, 327nm
[0060] Mobile phase: A. Volume concentration 0.08% phosphoric acid / acetonitrile B. Volume concentration 0.08% phosphoric acid / water
[0061] gradient:
[0062]
[0063] 2. Mixed standard preparation: with embodiment 1.
[0064] 3. Preparation of test solution: same as in Example 1
[0065] 4. Results: After determination, the test product contained baicalin 14.386mg / mL, forsythin 0.758mg / mL, chlorogenic acid 1.288mg / mL, neochlorogenic acid 0.98mg / mL, cryptochlorogenic acid 0.849mg / mL.
Embodiment 3
[0067] method of execution
[0068] 1. Analytical conditions: with embodiment 1.
[0069] 2. The preparation of need testing solution: with embodiment 1.
[0070] 3. Preparation of mixed standards: 1 mg / mL baicalein, wogonin, wogonin, scutellarin A, chlorogenic acid, isochlorogenic acid A, cryptochlorogenic acid, 3,4-dicaffeoyl Quinic acid, 1,3-dicaffeoylquinic acid, 4,5-dicaffeoylquinic acid, luteolin, rutin, forsythiaside, forsythialin, betulinic acid standard solution, Analyzed directly after filtration through 0.22 μm organic membrane.
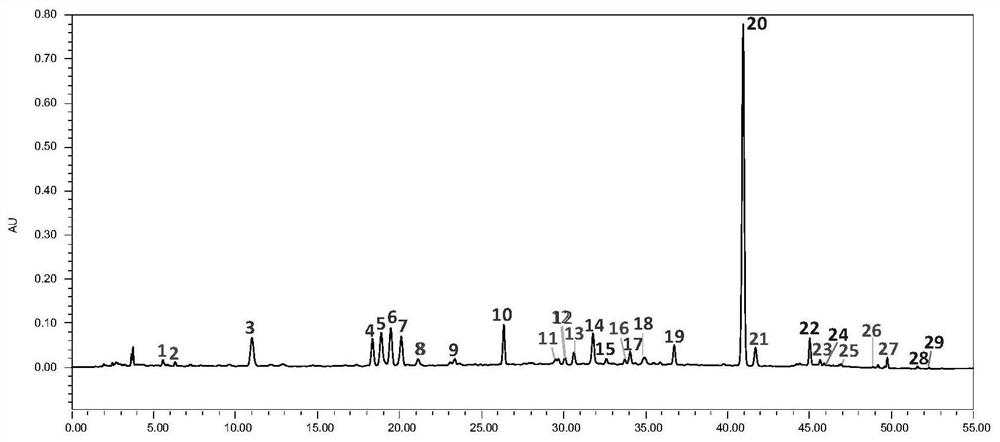
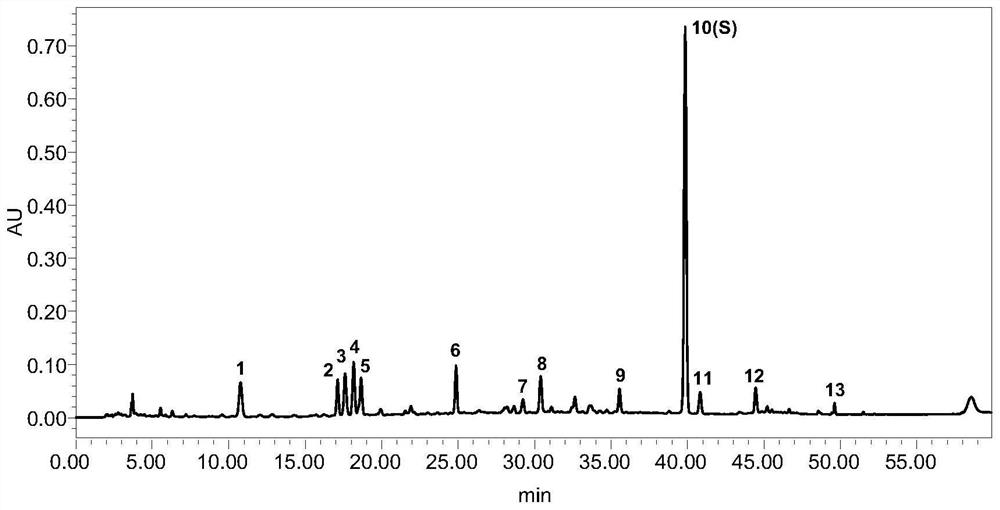
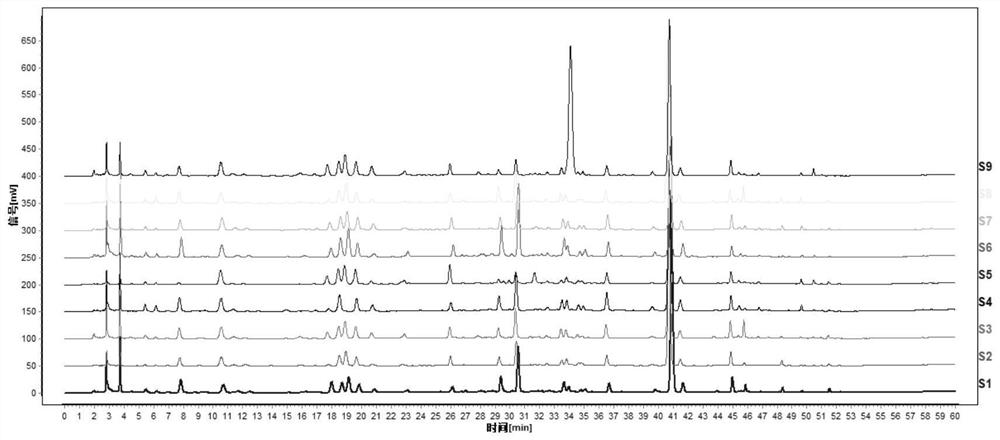
[0071] 4. Analysis results: Under the optimal separation conditions, the fingerprint peaks of Shuanghuanglian Oral Liquid were qualitatively analyzed using standard products, and the unknown principal component peaks were qualitatively analyzed by mass spectrometry, as shown in figure 1 , Shuanghuanglian oral liquid contains
[0072] 5. Neochlorogenic acid (3), Secologanoside* (4), chlorogenic acid (5), swertiamarin* (6), cryptochlorog...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Linear gradient | aaaaa | aaaaa |
| Linear gradient | aaaaa | aaaaa |
| Linear gradient | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com