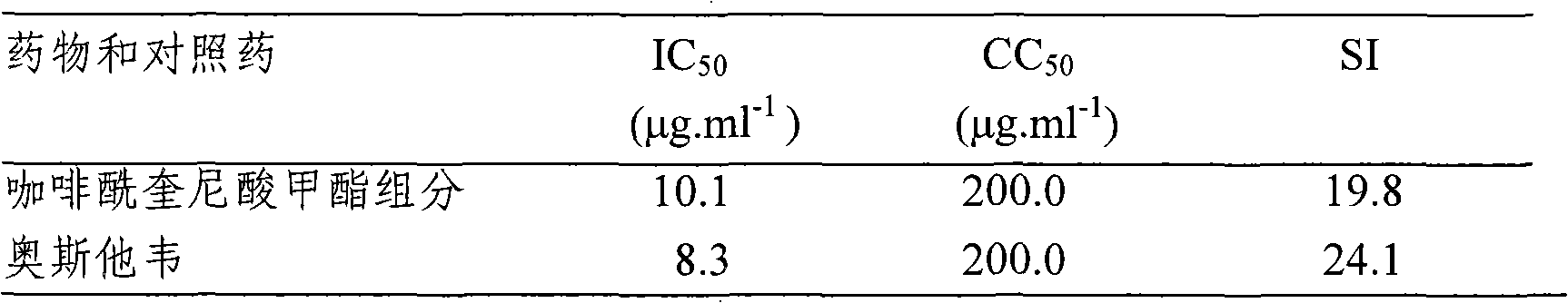Method for preparing dicaffeoylquinic acid methyl compound and composition thereof
A technology of caffeoylquinic acid methyl ester and caffeoylquinic acid, which is applied in the field of anti-influenza and anti-hepatitis compositions, and achieves the effects of mild reaction conditions, significant anti-influenza virus activity, and good yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
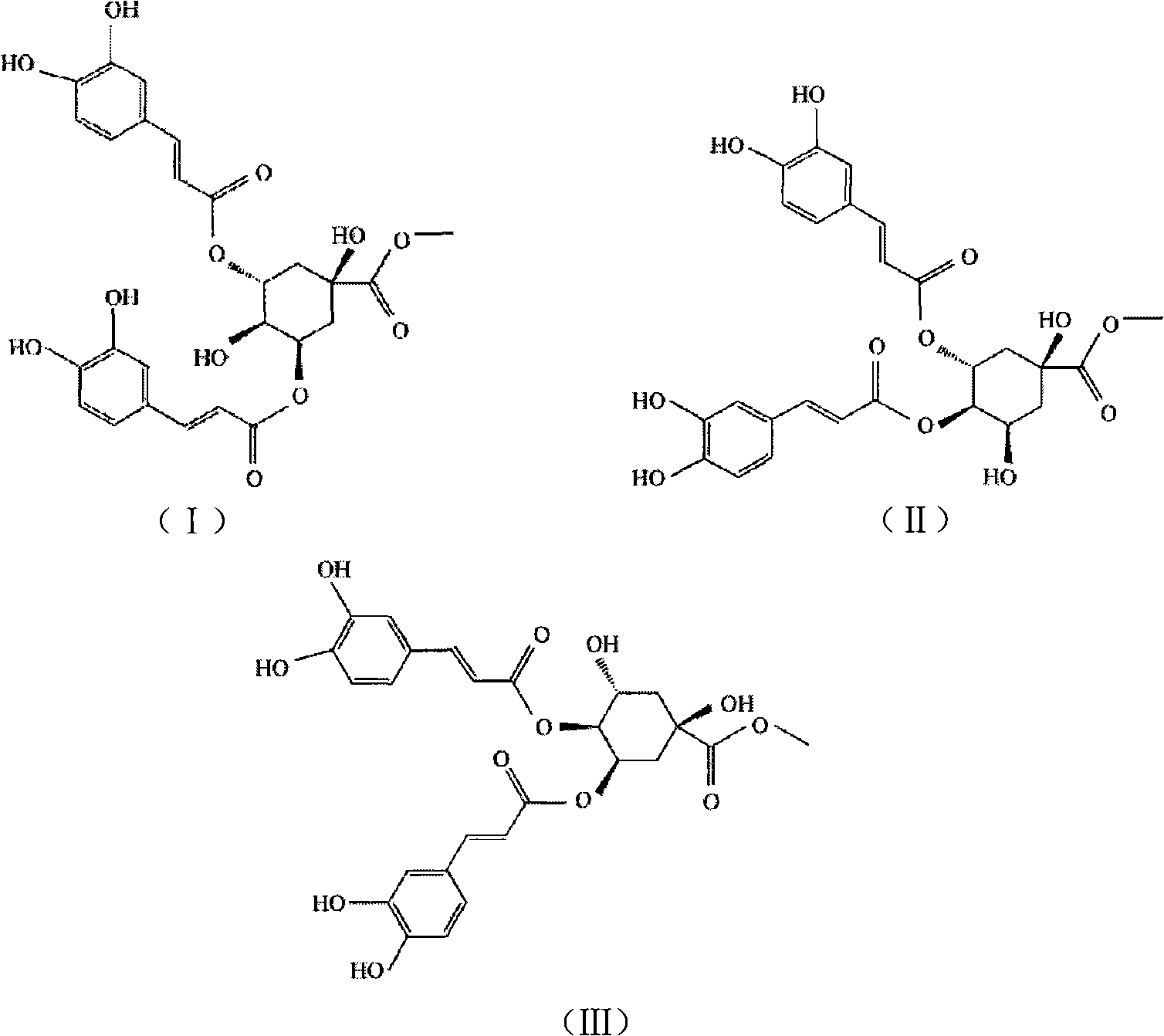
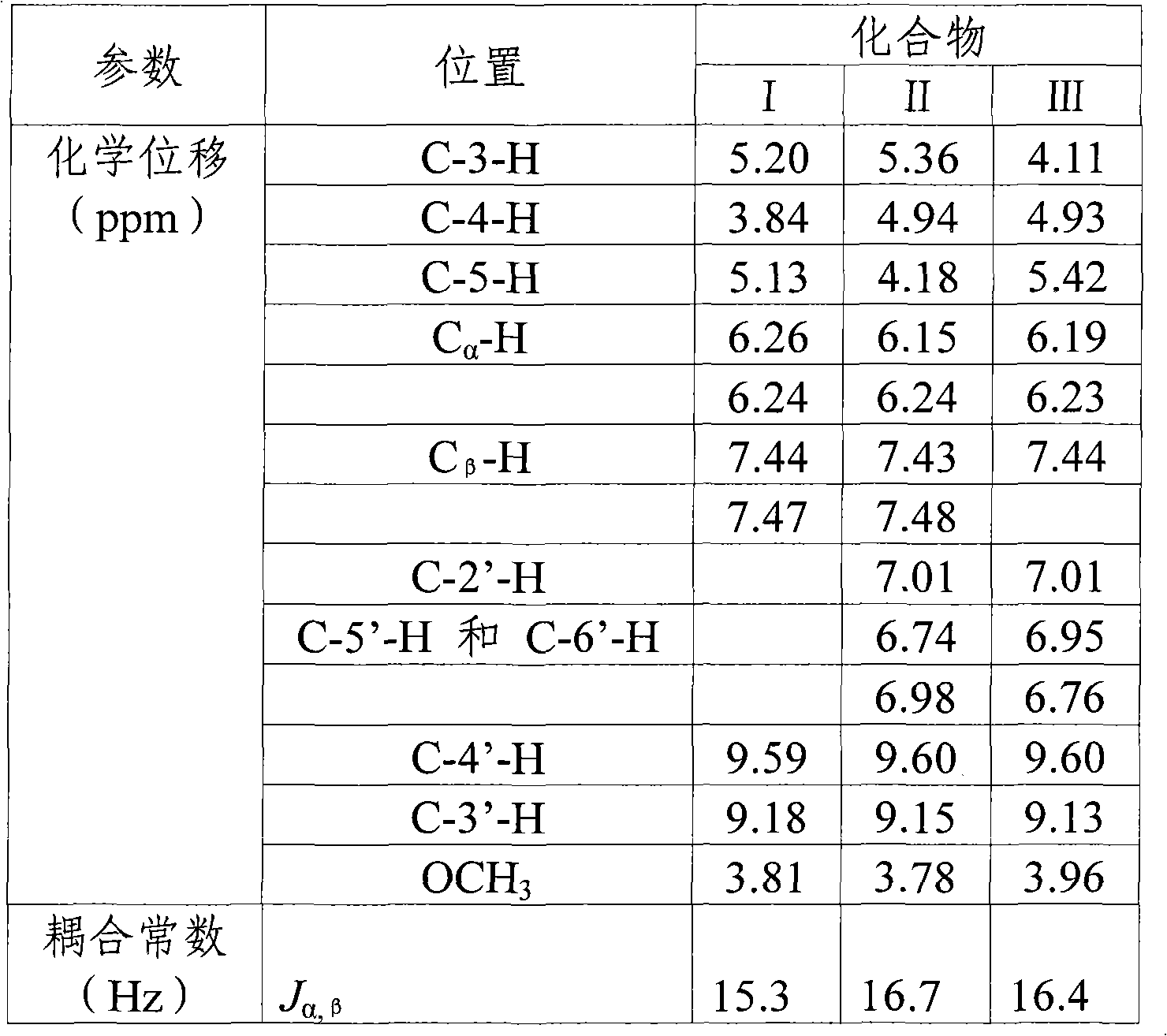
[0021] Preparation of dicaffeoylquinic acid methyl ester compound:
[0022] The dicaffeoylquinic acid methyl ester compound is prepared according to the following steps (taking Lonicera macranthoides Hand.-Mazz. dry medicinal materials in the mountain silver flower as an example):
[0023] Take 10kg of dried medicinal material, decoct twice with 10 times the amount of water, each time for 1 hour, combine the decoction, concentrate under reduced pressure to 0.5g crude drug / mL, and add it to the D101 macroporous adsorption resin (sample amount and resin volume The ratio is 1:2~4), first elute with 3 times column volume of water, and discard the water. Then use 5 times column volume of 10% ethanol to elute, and discard the 10% ethanol eluate. After elution with 5 times the column volume of 30-40% ethanol, collect the eluent, dry, add water to dissolve the residue to a concentration of 0.5-1g crude drug / ml, add dilute hydrochloric acid to adjust the pH to 1-4, and extract with et...
Embodiment 2
[0033] Tablet preparation of dicaffeoylquinic acid methyl ester compound composition:
[0034] prescription:
[0035] Crude drug (prepared in embodiment 1
[0036] Dicaffeoylquinic acid methyl esters) 15g
[0037] PVPK 30 1.5g
[0038] Microcrystalline Cellulose 4.5g
[0039] Low-substituted hydroxypropyl cellulose 0.45g
[0040] Sodium carboxymethyl starch 0.45g
[0041] Magnesium Stearate Appropriate amount
[0042]
[0043] Makes 100 pieces
[0044] Mix the above-mentioned pharmaceutical composition with auxiliary materials, add an appropriate amount of distilled water, stir and mix well, probe ultrasonically (4000r / min) for 5 minutes, and high-pressure milk (pressure 1000bar) for 10 laps to obtain a suspension; vacuum-dry and pass through an 80-mesh sieve , granulated with 70% ethanol, dried, added an appropriate amount of magnesium stearate and mixed evenly, and made into 100 tab...
Embodiment 3
[0046] Anti-influenza A virus effect of drugs
[0047] Test drug: caffeoylquinic acid methyl ester component (dicaffeoylquinic acid methyl ester compound prepared in Example 1, batch number: 20091106)
[0048] Experimental material: DMEM (Gibco company), cell maintenance medium except containing 1% fetal bovine serum (FBS, American Gibco product), other components are the same as DMEM culture medium. Hep2 cells, MDCK cells, influenza virus standard strain (A / PR8 / 34), influenza A virus (FluA, H1N1), provided by the Institute of Infectious Diseases of the PLA. 96-well cell culture plate, produced by Calif, USA.
[0049] Cytotoxicity test: Hep2 cells or MDCK cells were seeded in 96-well cell culture plates and placed in CO at 37°C 2 Cultivate in the incubator for 2 days. After the cells grow into a single layer, remove the culture medium, add 0.1ml maintenance solution half-diluted test drug solution, and set 0.1mL maintenance solution as a blank control. Continue to place in ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com