Quality control method for erigeron breviscapus (Vant.) hand-mazz.
A quality control method and technology of Asarum radix are applied in pharmaceutical formulations, medical preparations containing active ingredients, antipyretics, etc., which can solve the problems of not fully reflecting the quality, complex chemical composition, poor separation effect, etc., and achieve perfection. Quality control system, improved separation efficiency, easy operation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
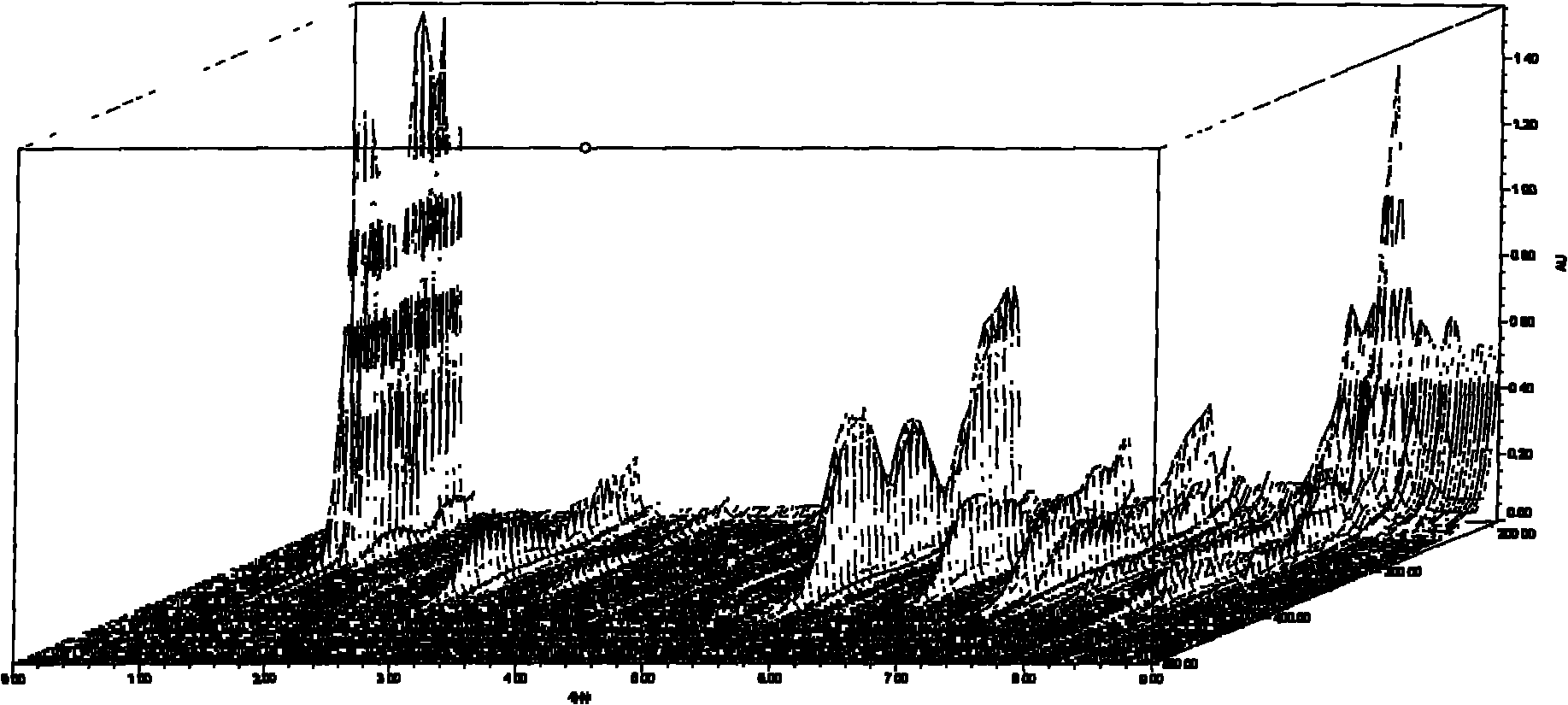
[0037] Below in conjunction with specific embodiment, further illustrate the present invention. The following examples are only for illustrating the present invention and it should not be construed that the scope of the above-mentioned subject matter of the present invention is limited to the following examples. In addition, it should be understood that after reading the teachings of the present invention, those skilled in the art can make various changes or modifications to the present invention, and these equivalent forms also fall within the scope defined by the appended claims of the present application. At the same time, for the purpose of simplicity and clarity, the description of known technologies is appropriately omitted below, so as not to affect the description of the technical solution with unnecessary details. Example 1 Establishment of the UPLC fingerprint of Erigeron breviscapus medicinal material
[0038] 1 Instruments and reagents
[0039] 1.1 Instrument
...
Embodiment 2
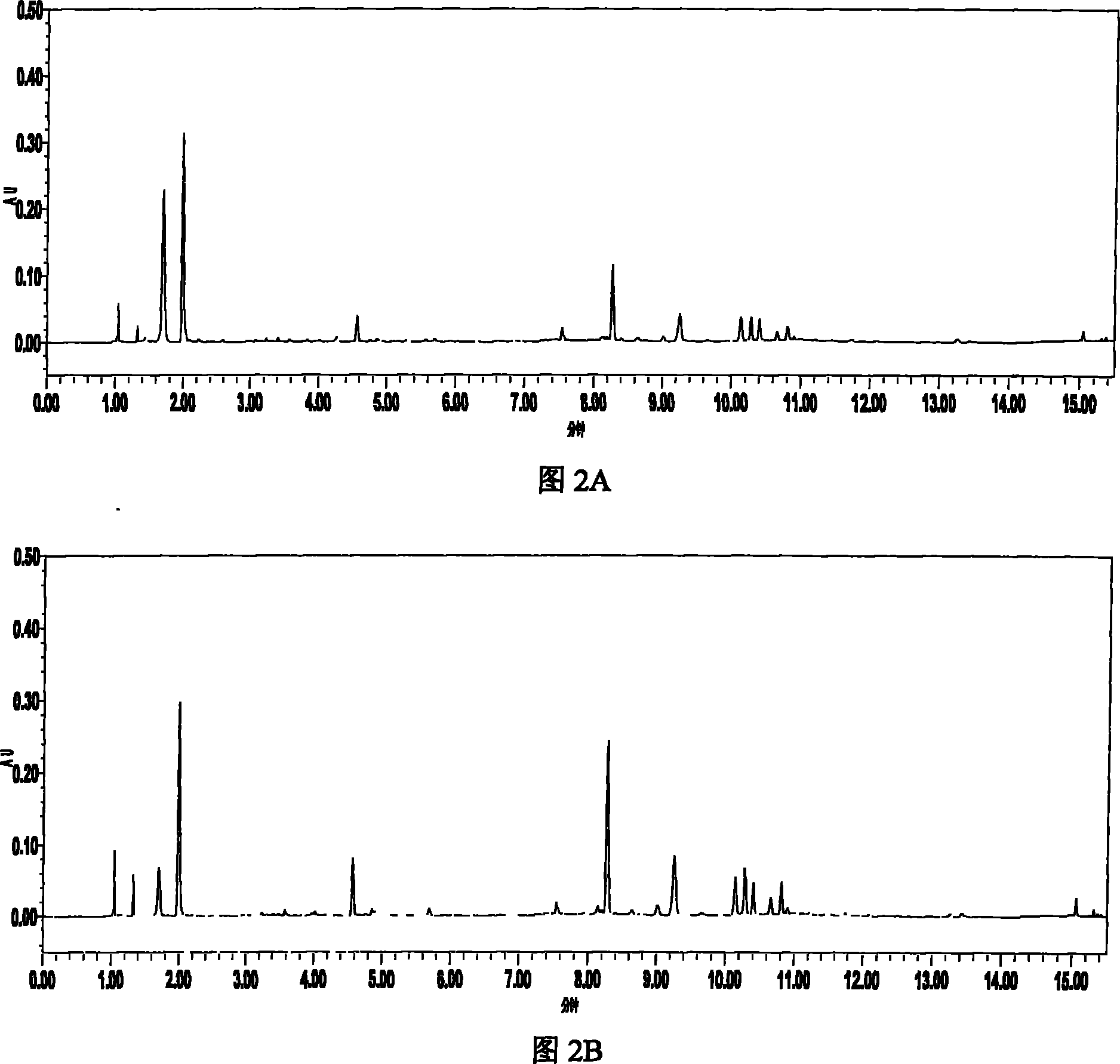
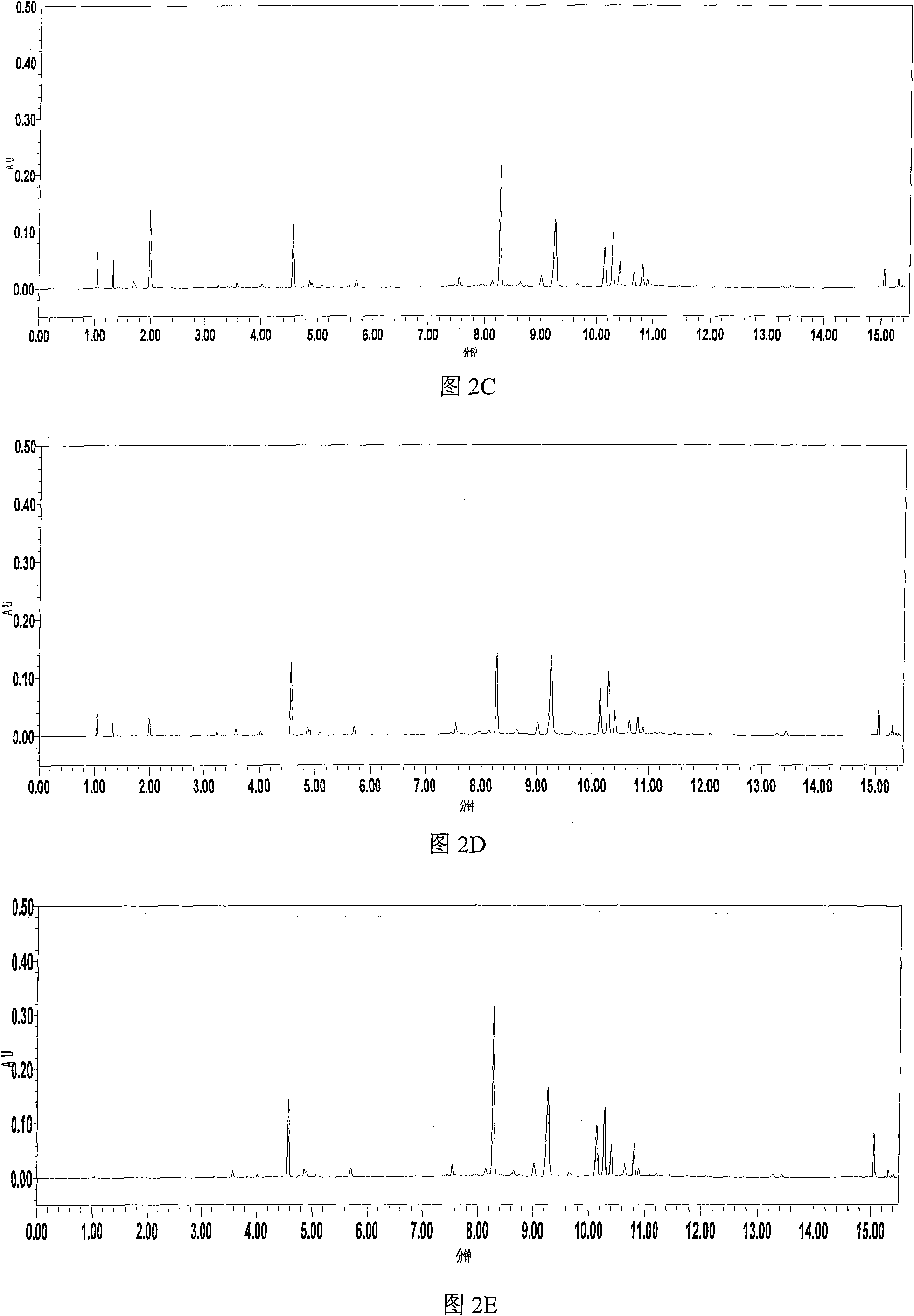
[0069] Example 2 UPLC-PDA method for simultaneous determination of the contents of 8 components in Erigeron breviscapus medicinal material
[0070] 1 solution preparation
[0071] 1.1 Test solution: Accurately weigh about 0.5g of Erigeron breviscapus sample crushed through a 40-mesh sieve, accurately add 50mL of 50% methanol, weigh it, heat to reflux for 2 hours, let it cool, weigh it again, and add 50% methanol Make up for the lost weight, shake well, centrifuge, take the supernatant and filter it with a 0.22 μm microporous membrane, and take the subsequent filtrate as the test solution.
[0072] 1.2 Reference substance solution: Accurately weigh 12.58mg of reference substances, scutellarin, 6.12mg of chlorogenic acid, 2.18mg of scutellarin (purity 95.3%), 11.15mg of scutellarin (purity 97.1%), and 2.43mg of scutellarin (purity 96.1%) , 3,5-O-dicaffeoylquinic acid (purity 94.6%) 9.63mg, scutellarin 4.37mg, 4,5-O-dicaffeoylquinic acid (purity 96.2%) 4.22mg, placed in 25mL In...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com