Preparation method of boric-acid functionalized porous adsorbent
A porous adsorbent, functionalized technology, applied in chemical instruments and methods, solid adsorbent liquid separation, separation methods, etc., to achieve the effect of reducing secondary environmental pollution, multiple binding sites, and less pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
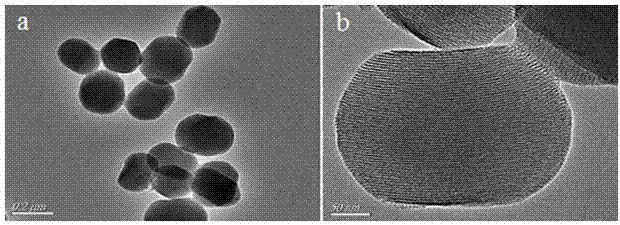
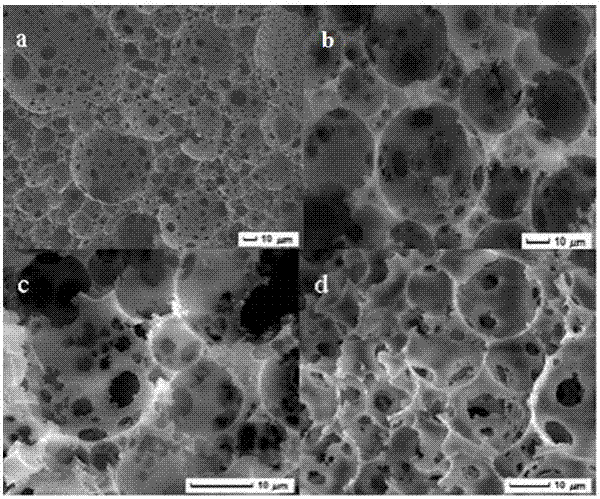
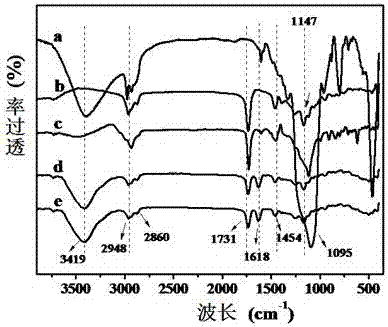
[0027] (1) Preparation of boric acid functionalized mesoporous nanoparticles (BA-MSNs):
[0028] First, 1.37g of 3-aminophenylboronic acid and 2.36g of 3-glycidyloxypropyltriethoxysilane were added to a 25mL round bottom flask, then 10mL of tetrahydrofuran was slowly added, and nitrogen gas was introduced to remove oxygen for 15min, then at 25 o C under reaction for 12h. Finally, the mixed solution was removed by rotary evaporation, and the resulting brown oily substance was dissolved in 10 mL of methanol solution to obtain a concentration of 1.0 mmol mL -1 APBA-GPTES solution.
[0029] Subsequently, 0.3 g of cetyltrimethylammonium bromide and 0.1 g of sodium hydroxide were dissolved in 150 mL of deionized water, heated and stirred to 75 o C, then add 1.25mL tetraethyl silicate and 100µL APBA-GPTES solution dropwise, after stirring continuously for 2h, centrifuge and wash the precipitate 5 times with ethanol. Finally, the product obtained at 50 o Boronic acid-functionaliz...
Embodiment 2
[0038] (1) Preparation of boric acid functionalized mesoporous nanoparticles (BA-MSNs):
[0039] First, 1.37g of 3-aminophenylboronic acid and 2.36g of 3-glycidyloxypropyltriethoxysilane were added to a 25mL round bottom flask, then 10mL of tetrahydrofuran was slowly added, and nitrogen gas was introduced to remove oxygen for 15min, then at 25 o C under reaction for 12h. Finally, the mixed solution was removed by rotary evaporation, and the resulting brown oily substance was dissolved in 10 mL of methanol solution to obtain a concentration of 1.0 mmol mL -1 APBA-GPTES solution.
[0040] Subsequently, 0.2 g of cetyltrimethylammonium bromide and 0.05 g of sodium hydroxide were dissolved in 150 mL of deionized water, heated and stirred to 70 o C, then add 1.0 mL tetraethyl silicate and 50 µL APBA-GPTES solution drop by drop, after stirring continuously for 1 h, centrifuge and wash the precipitate 5 times with ethanol. Finally, the product obtained at 50 o Boronic acid-functi...
Embodiment 3
[0046] (1) Preparation of boric acid functionalized mesoporous nanoparticles (BA-MSNs):
[0047] First, 1.37g of 3-aminophenylboronic acid and 2.36g of 3-glycidyloxypropyltriethoxysilane were added to a 25mL round bottom flask, then 10mL of tetrahydrofuran was slowly added, and nitrogen gas was introduced to remove oxygen for 15min, then at 25 o C under reaction for 12h. Finally, the mixed solution was removed by rotary evaporation, and the resulting brown oily substance was dissolved in 10 mL of methanol solution to obtain a concentration of 1.0 mmol mL -1 APBA-GPTES solution.
[0048] Subsequently, 0.4g of cetyltrimethylammonium bromide and 0.15g of sodium hydroxide were dissolved in 150mL of deionized water, heated and stirred to 80 o C, then add 1.5mL tetraethyl silicate and 150µL APBA-GPTES solution dropwise, after stirring continuously for 3h, centrifuge and wash the precipitate 5 times with ethanol. Finally, the product obtained at 50 o Boronic acid-functionalized ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Adsorption capacity | aaaaa | aaaaa |
| Adsorption | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com