Marker composed of tissue snoRNA and kit used for predicting liver cancer recurrence risk
A marker and kit technology, applied in the field of detection kits for assessing the recurrence risk of hepatocellular carcinoma patients after undergoing resection, can solve the problems of difficulty in detection implementation, poor usability, and few of them, so as to avoid excessive treatment and achieve mature experimental methods. , the effect of simple detection process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1: Collection and preparation of tumor tissue samples
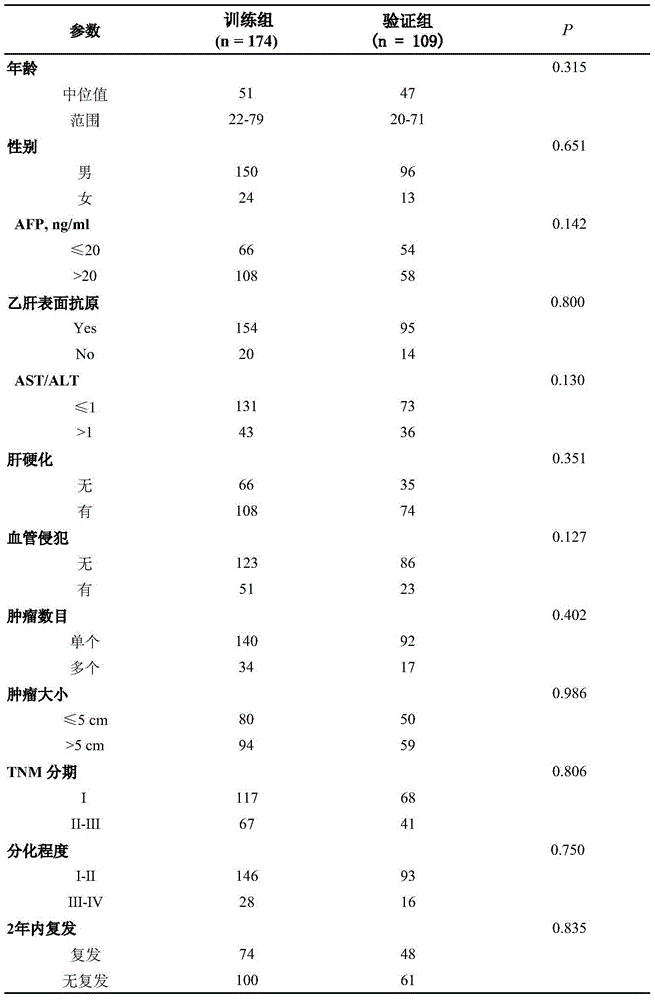
[0033] The inventors collected tumor tissue samples from patients with liver cancer (HCC) who underwent liver cancer resection between January 2006 and November 2011. These populations met the following inclusion criteria, and according to the principle of gender and age matching, set liver cancer and its control samples. Inclusion criteria: (1) primary liver cancer, newly diagnosed and underwent radical surgical resection; (2) aged between 18 and 80 years old; (3) without extrahepatic metastasis at the time of diagnosis; (4) without liver cancer before operation Other malignant diseases, and no anti-cancer treatment before postoperative recurrence; (5) No symptoms of severe organ disorders after postoperative.
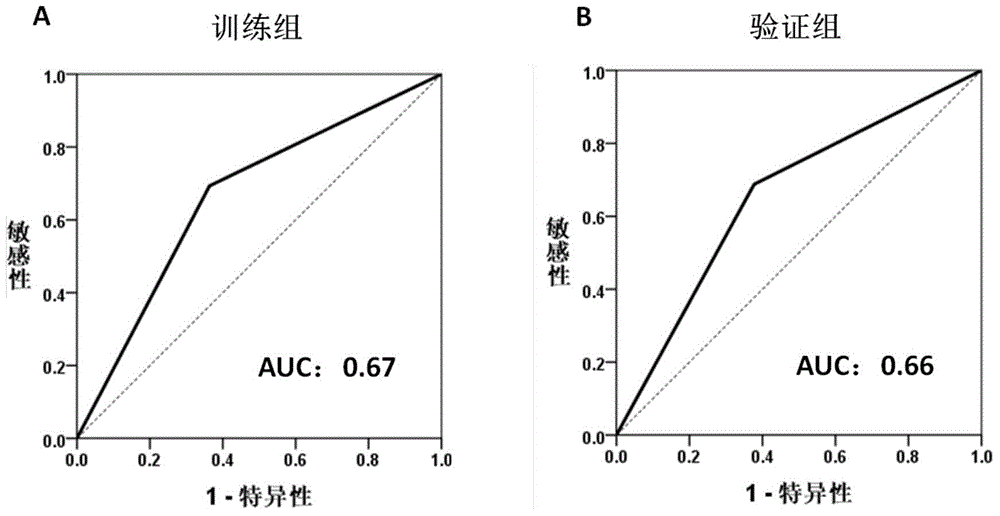
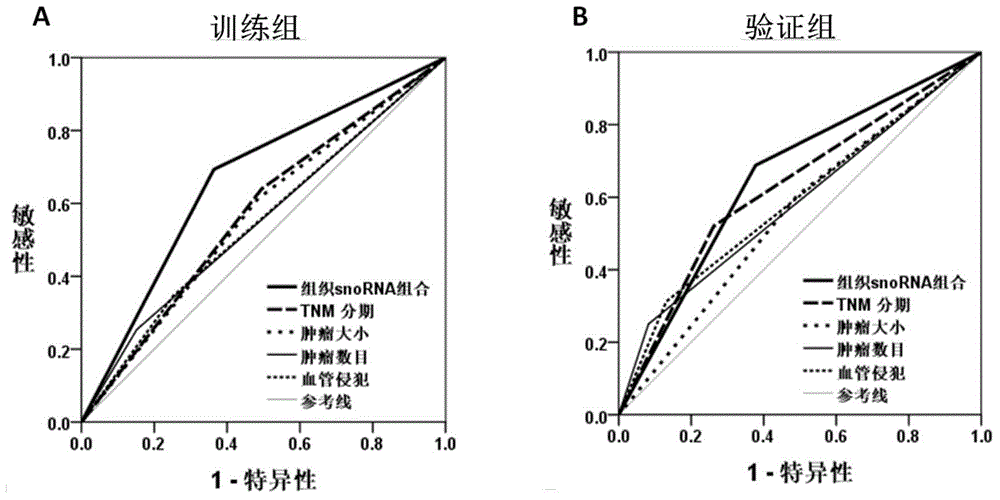
[0034] Training group: 174 cases of HCC tumor tissue specimens who underwent liver cancer resection between January 2006 and December 2009.
[0035] Validation group: 109 cases of HCC tumor tissu...
Embodiment 2
[0039] Embodiment 2: gene chip and its data analysis
[0040] The inventor selected 5 cases of liver cancer tissue and 3 cases of normal liver tissue (paratumor liver tissue of hepatic hemangioma) for gene chip screening. These specimens were obtained from patients undergoing radical resection of HCC or resection of hemangioma in 2005-2006, all were confirmed by pathology, and were frozen in liquid nitrogen immediately after resection.
[0041] The chip was produced by CapitalBio Corp, and a total of 281 snoRNA levels were detected. After calibration of the obtained raw data, the inventors used the Significant Analysis of Microarray (SAM) analysis method to select differential snoRNAs, and finally screened and obtained 28 candidate snoRNAs for subsequent verification: ACA3, U15a, U19, ACA21, ACA31, U31, U35B , ACA38, U35b, snR38c, U42B, U44, ACA52, U52, U53, U54, U58b, U60, ACA61, U70, U75, U78, U81, U106, HBII-142, HBII-296, HBII-202, HBII-420 .
Embodiment 3
[0042] Embodiment 3: Real-time fluorescent quantitative PCR detects the level of snoRNAs in the specimens of the training group
[0043] 1. Tissue RNA Extraction
[0044] The present invention adopts Trizol reagent to extract, and specific steps are as follows: (1) For the tissue preserved in RNALater or liquid nitrogen, add 1ml Trizol ratio lysis cell per 50mg tissue; (2) Transfer Trizol lysate to 1.5ml Rnase free EP Shake and mix in the tube, place at room temperature for 5 minutes, add 1 / 5 of the lysate volume of chloroform, shake and mix, centrifuge at 12,000 g at 4°C for 15 minutes; absorb the supernatant, add an equal volume of isopropanol, place at room temperature for 10 minutes, and store at 4°C, Centrifuge at 12,000 g for 30 min; (3) Discard the supernatant, wash the precipitate twice with 70% ethanol, pour off the supernatant carefully, add an appropriate amount of DEPC water to dissolve the RNA after the alcohol evaporates, and store at -80°C for later use.
[004...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com