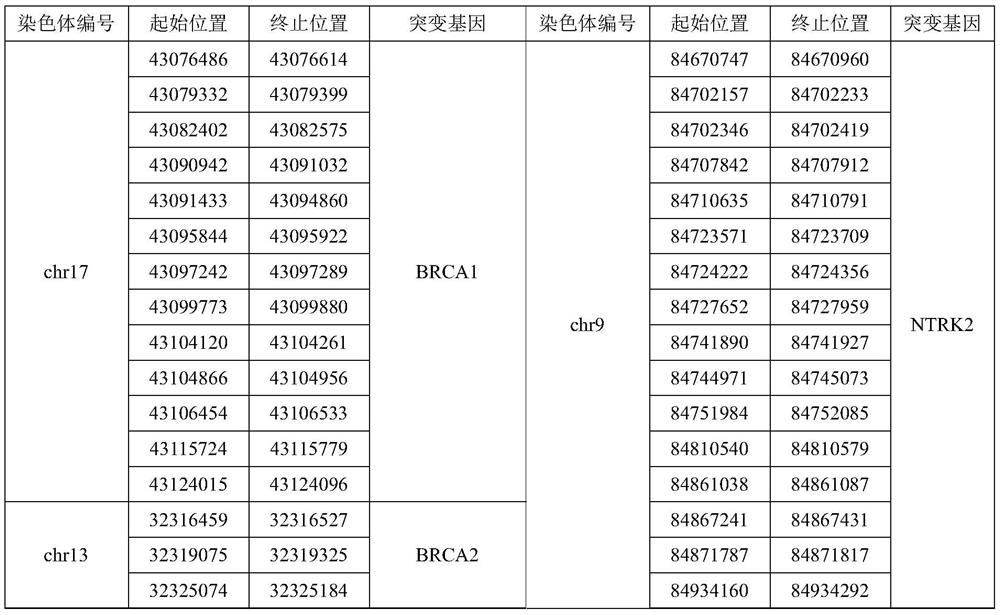
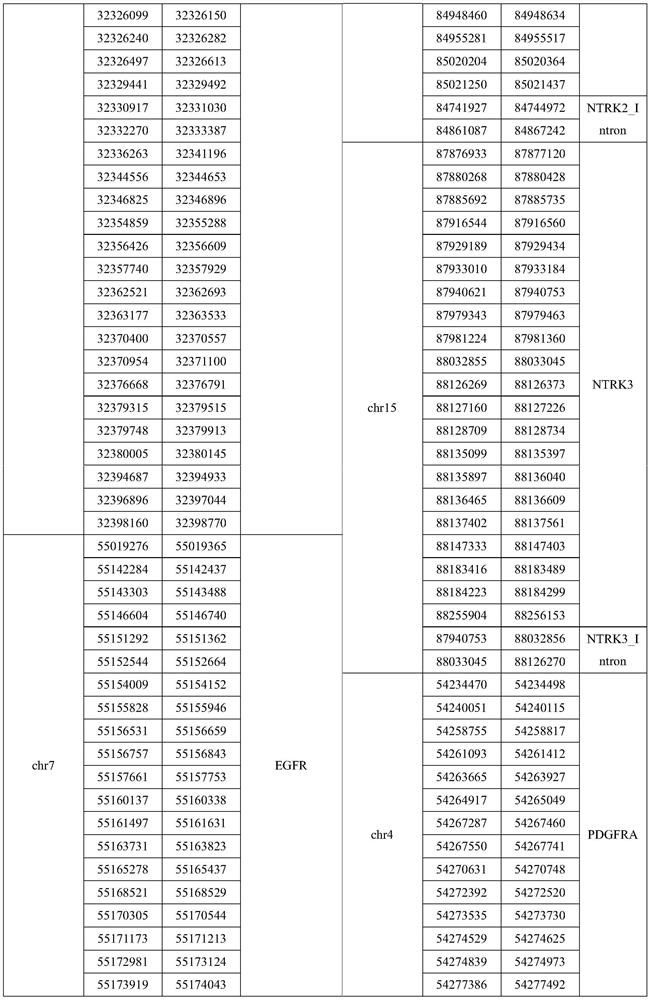
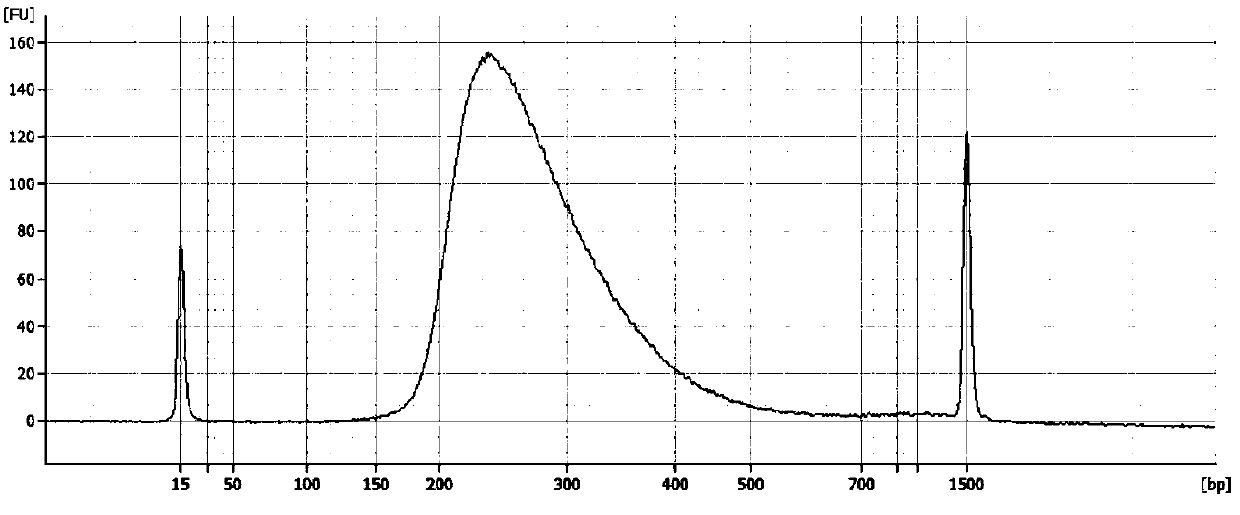
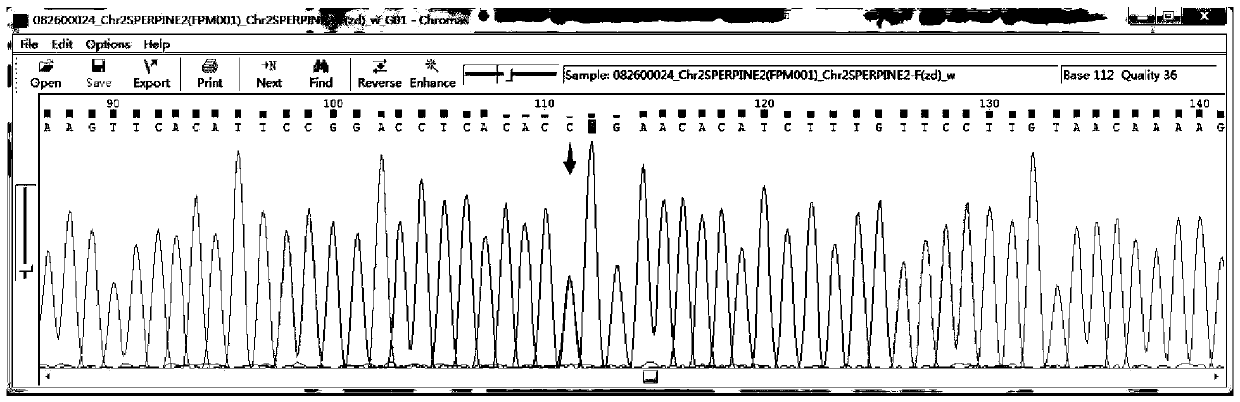
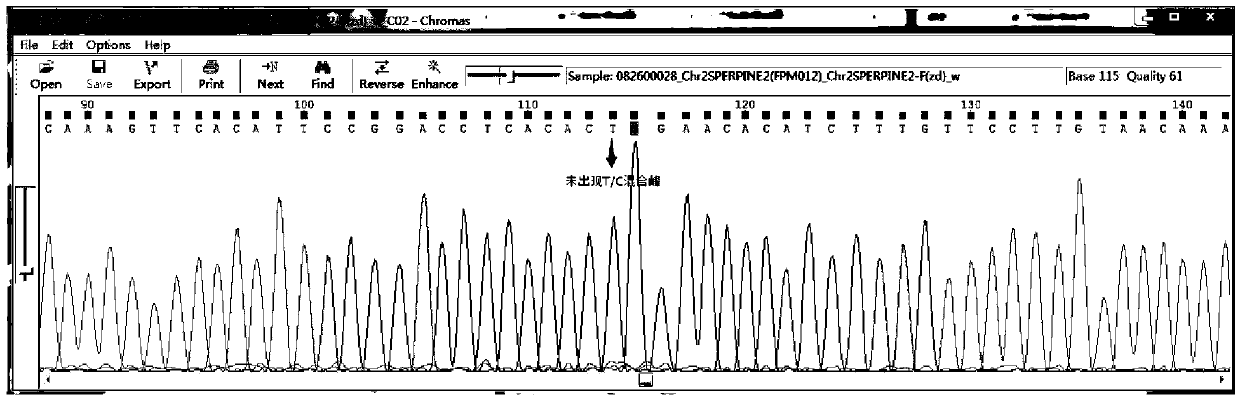
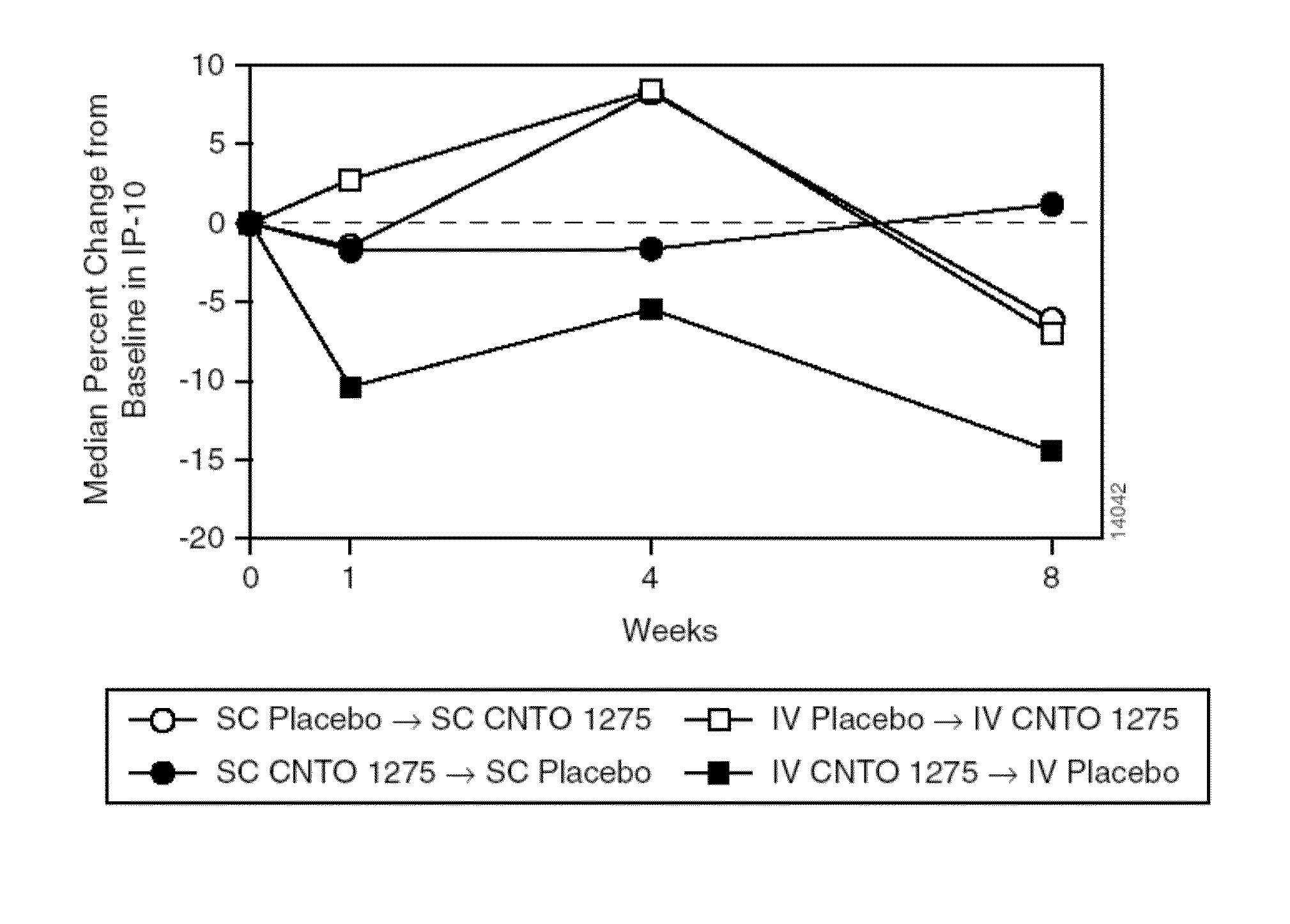
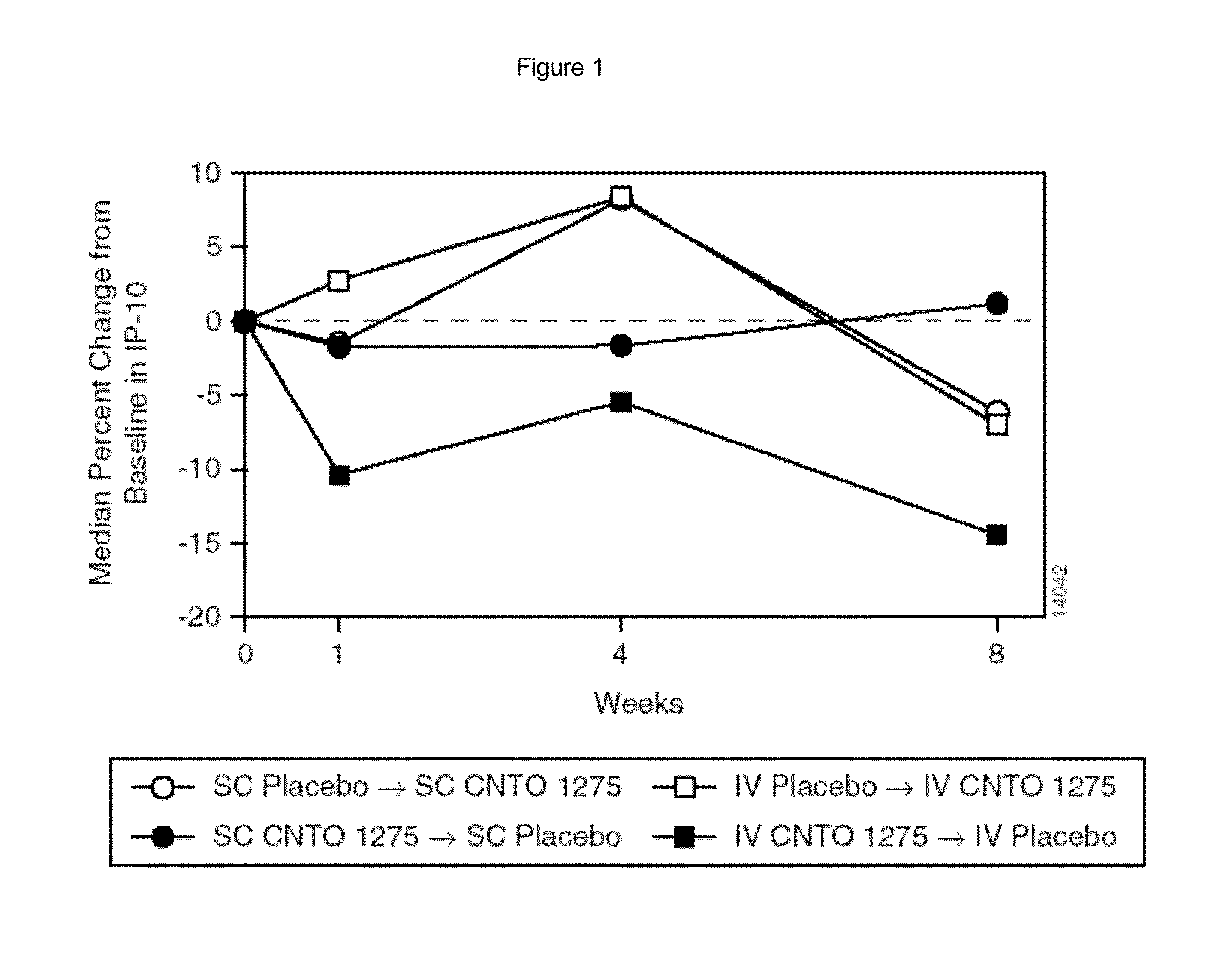
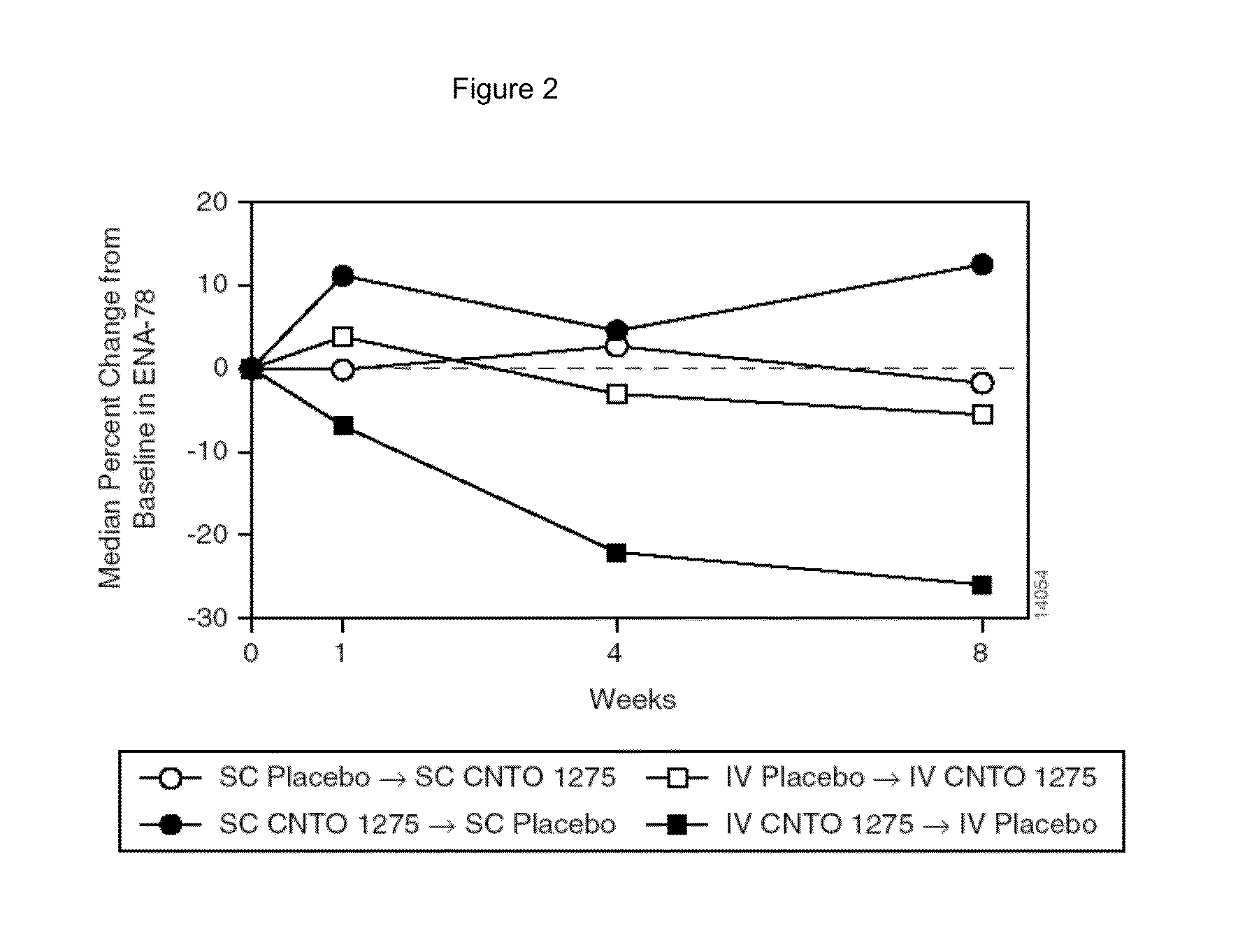
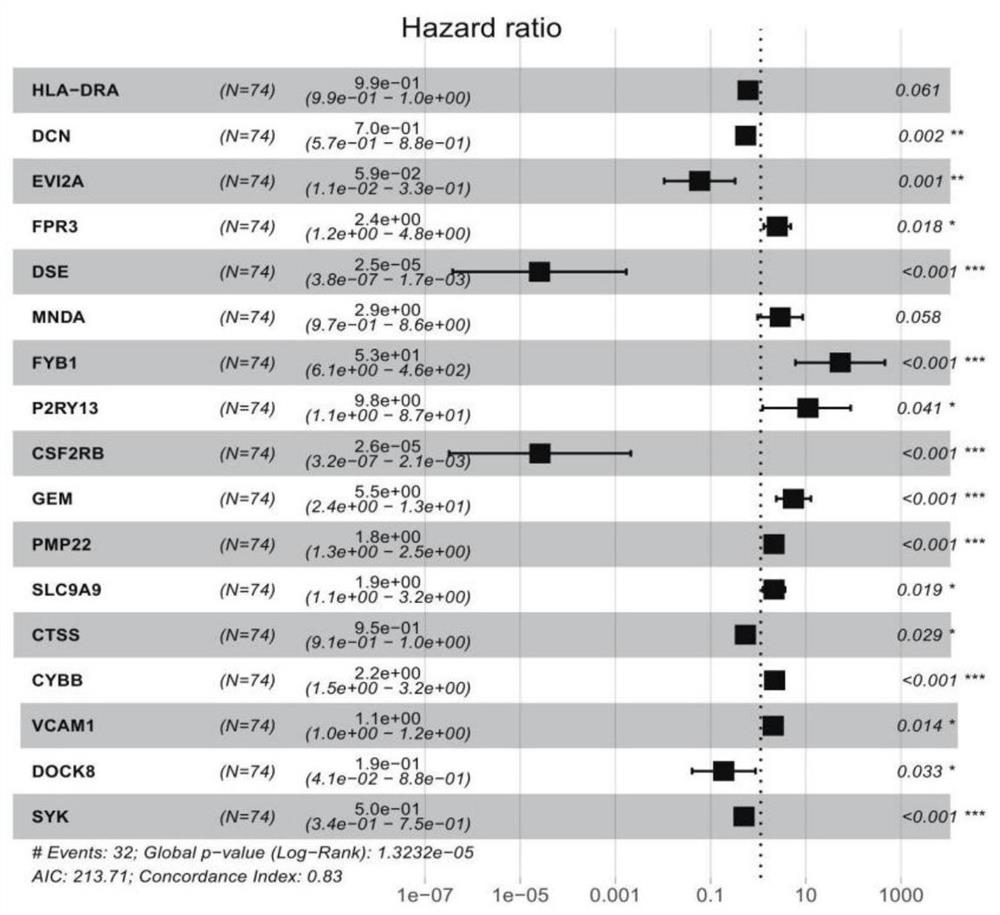
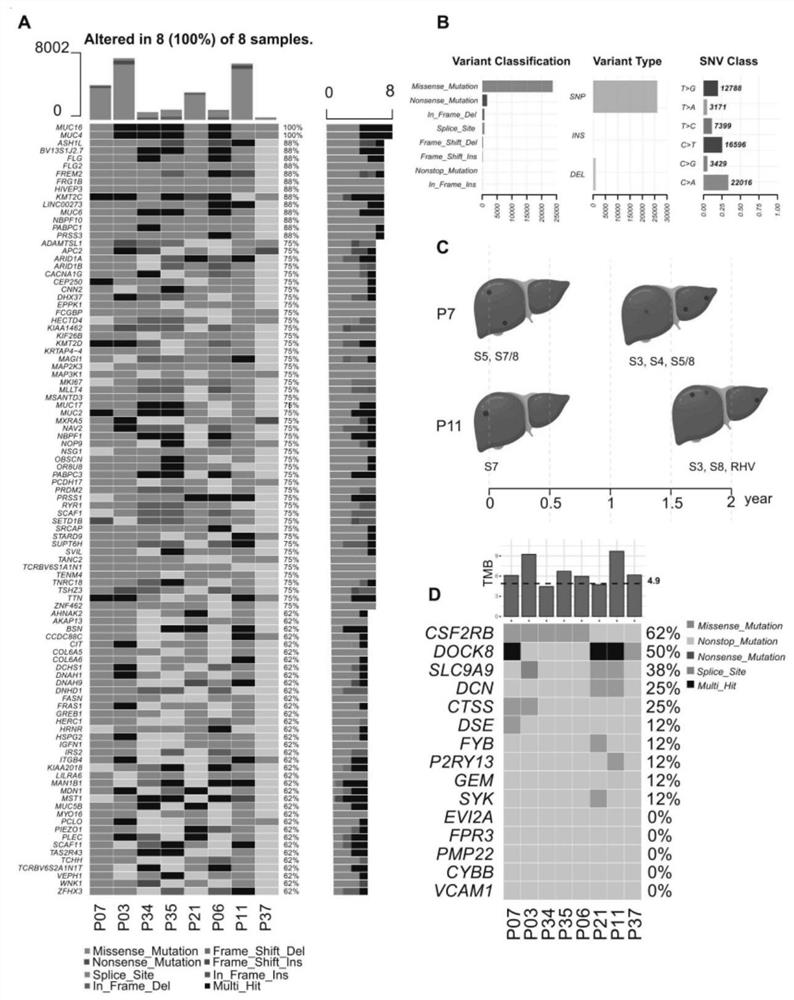
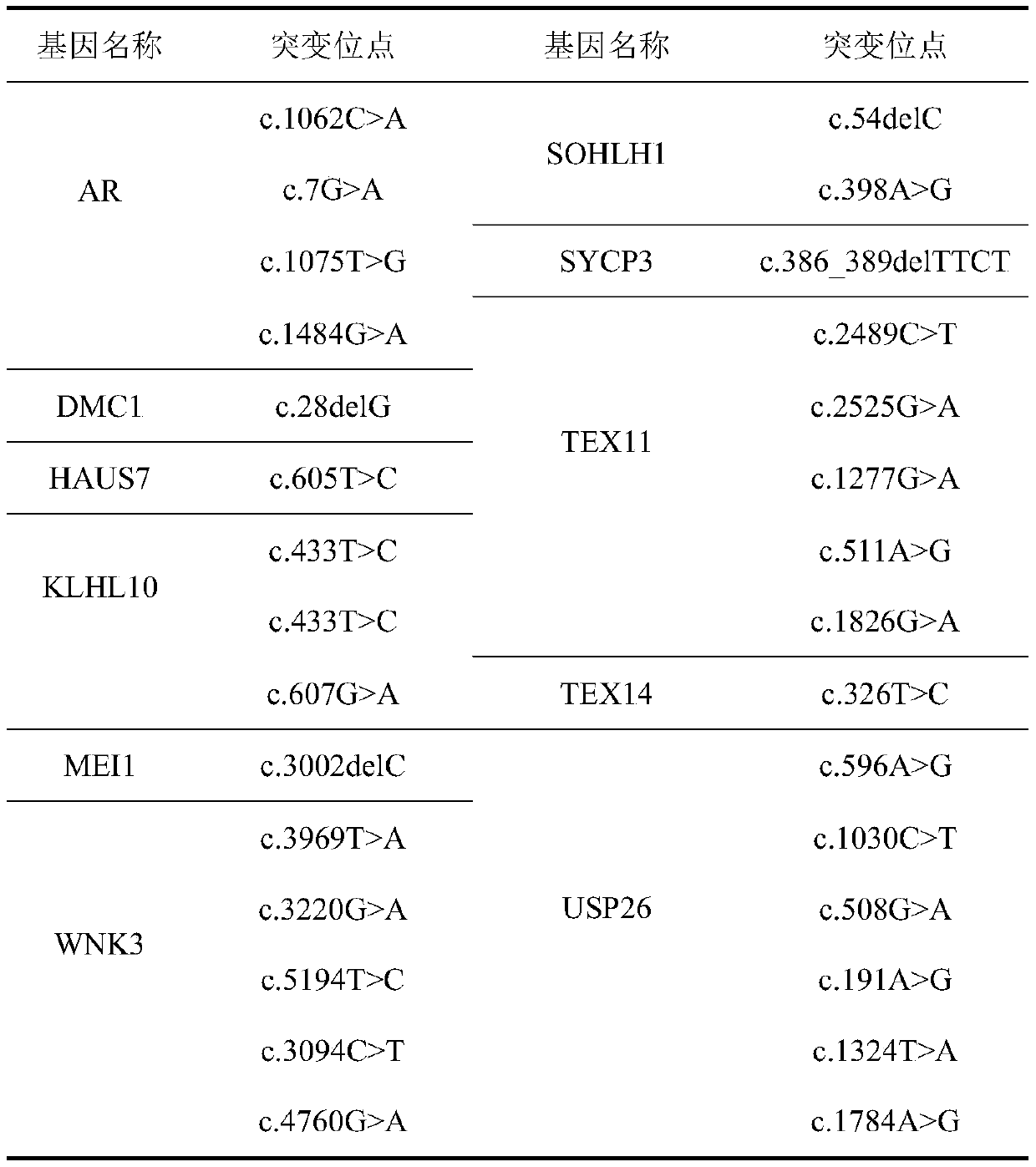
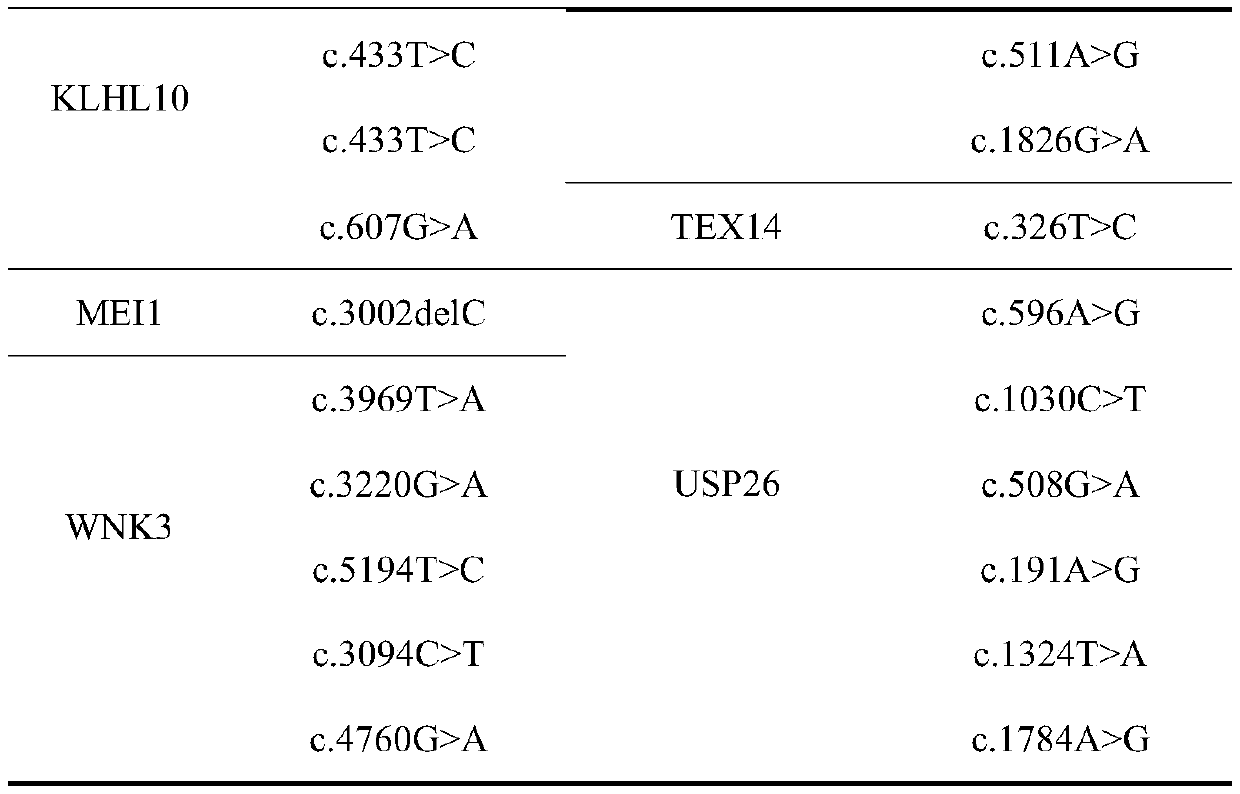
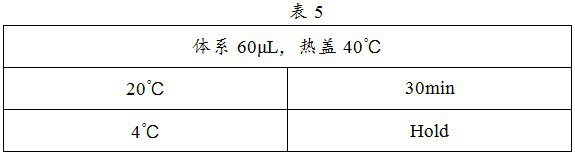
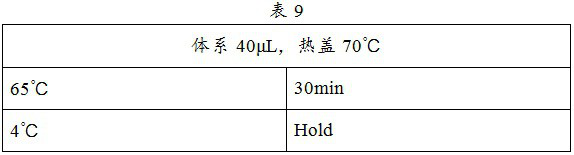
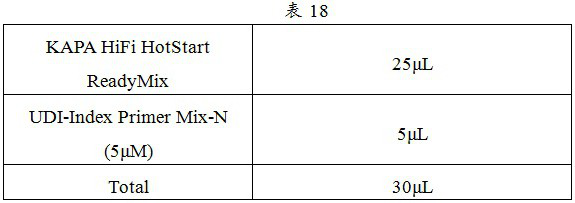
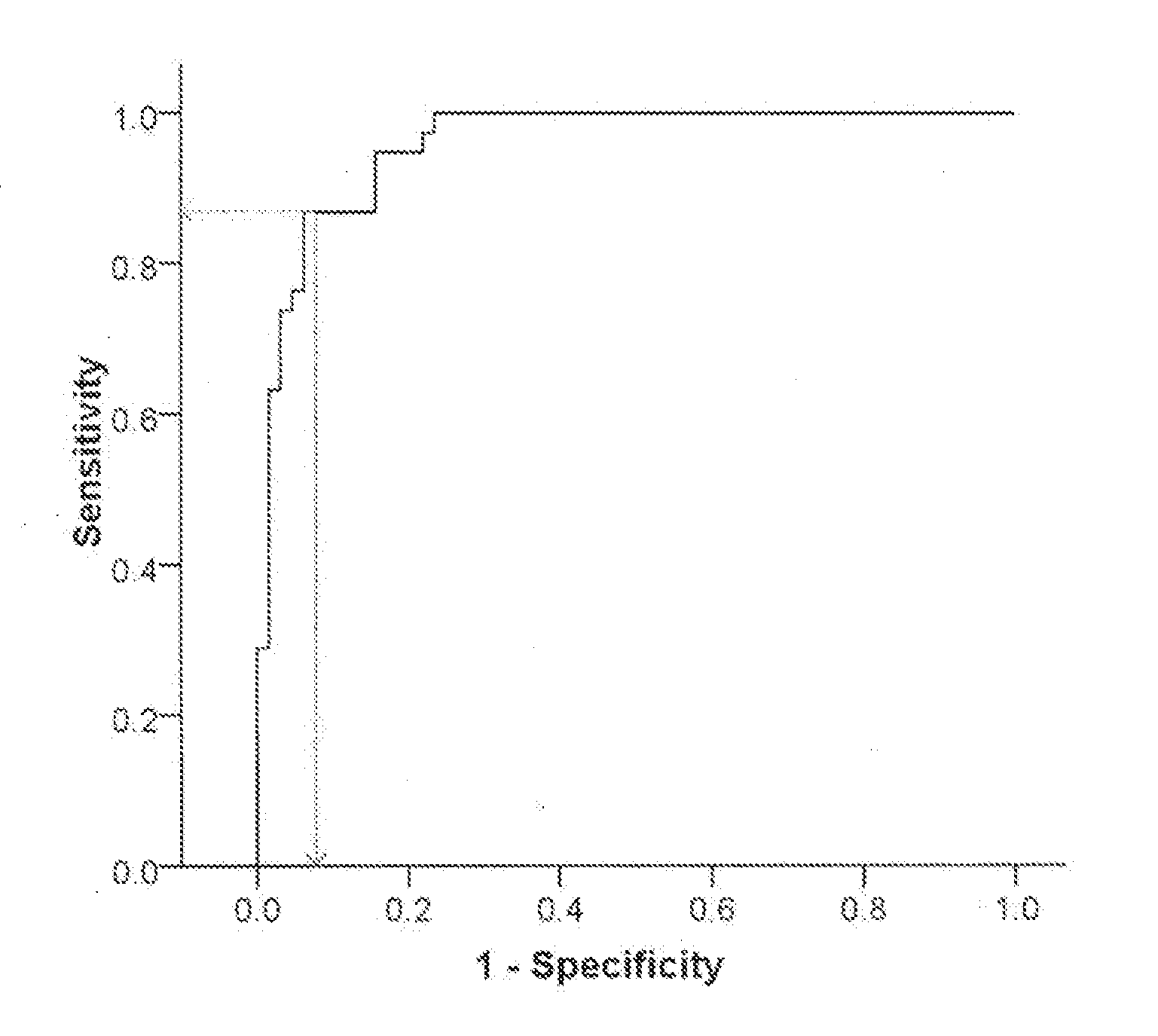Patents
Literature
59 results about "Gene panel" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Gene Panels. A gene panel is a test that analyzes multiple genes at once for cancer-associated mutations. “Gene panels offer a middle ground between sequencing just a single gene like BRCA1 that we are certain is involved in disease risk, and sequencing every gene in the genome.
Method for the development of gene panels for diagnostic and therapeutic purposes based on the expression and methylation status of the genes
InactiveUS20020137086A1Fast and reliable diagnosis and therapyPowerful toolBioreactor/fermenter combinationsNervous disorderCytosineTherapeutic intent
A method for the development of gene panels for diagnostic and therapeutic purposes comprising the steps of: (a) isolating at least one biological sample from each of at least two groups of biological material containing mRNA and / or proteins; (b) analyzing the expression level of at least one gene in the at least one biological sample; (c) selecting the gene(s) exhibiting a different expression level between the at least two groups of biological material, whereby a first knowledge base is generated; (d) analyzing the level of cytosine methylation in the methylation relevant regions of at least one gene of at least one of the biological samples of step (a), wherein the gene is selected on the basis of the first knowledge base; (e) selecting the gene(s) exhibiting a different level of cytosine methylation between the at least two groups of biological material, whereby a second knowledge base is generated; and (f) adding selected genes from the second knowledge base to a gene panel.
Owner:EPIGENOMICS AG
Markers for cancer prognosis and therapy and methods of use
InactiveUS20130042333A1Adverse side effectLow costBiocideMicrobiological testing/measurementAdjuvantTreatment field
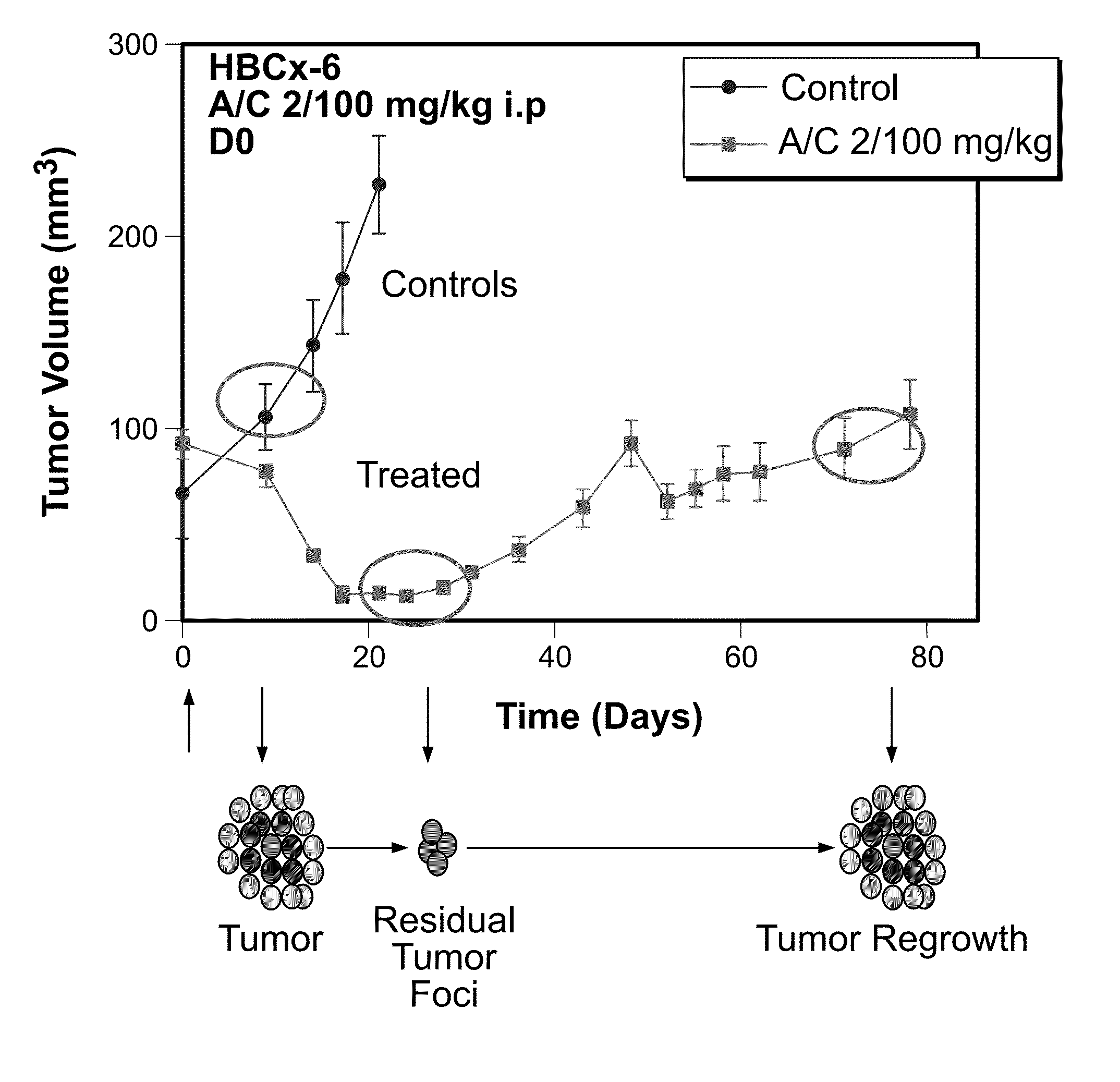
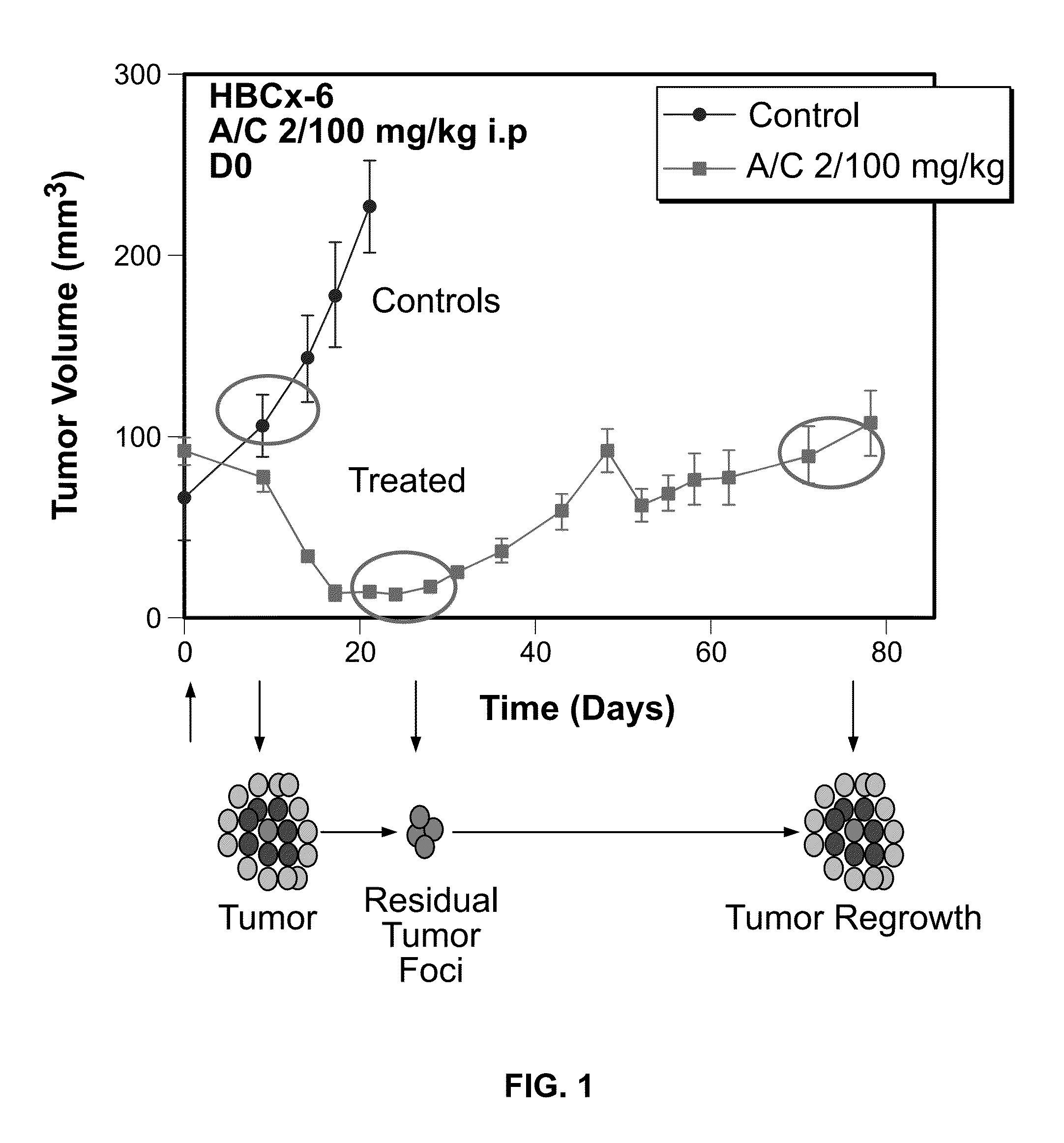
The invention relates generally to the field of cancer prognosis and treatment. More particularly, the present invention relates to methods and compositions that utilize a particular panel of gene products (“biomarkers”) and their differential expression patterns (“expression signatures”), wherein the expression patterns correlate with responsiveness, or lack thereof, to chemotherapy treatment. The invention is based on the identification of a specific set of biomarkers that are differentially expressed in chemotherapy-treated tumors and which are useful in predicting the likelihood of a therapeutic response, including residual disease persistence and subsequent tumor recurrence in cancer patients receiving chemotherapy. The gene panel is also useful in designing specific adjuvant modalities with improved therapeutic efficiency. Also disclosed are methods for characterizing tumors according to expression of the biomarkers described herein.
Owner:GEN TECH
Markers and Methods for Assessing and Treating Ulcerative Colitis and Related Disorders Using a 43 Gene Panel
InactiveUS20080293582A1Avoid actionInhibit productionMicrobiological testing/measurementLibrary screeningMedicineUlcerative colitis
A method for prognostic or diagnostic assessment of a gastrointestinal-related disorder, such as ulcerative colitis, in a subject correlates the presence, absence, and / or magnitude of a gene in a sample with a reference standard to determine the presence and / or severity of the disorder, and / or the response to treatment for the disorder. The method enables identification of the effectiveness of candidate therapies.
Owner:CENTOCOR ORTHO BIOTECH
Gene panel used for detecting breast cancer gene mutation, detection method used for detecting breast cancer gene mutation and application of gene panel
InactiveCN111647648AComprehensive immunotherapyComprehensive treatmentMicrobiological testing/measurementSequence analysisHuman DNA sequencingGenome human
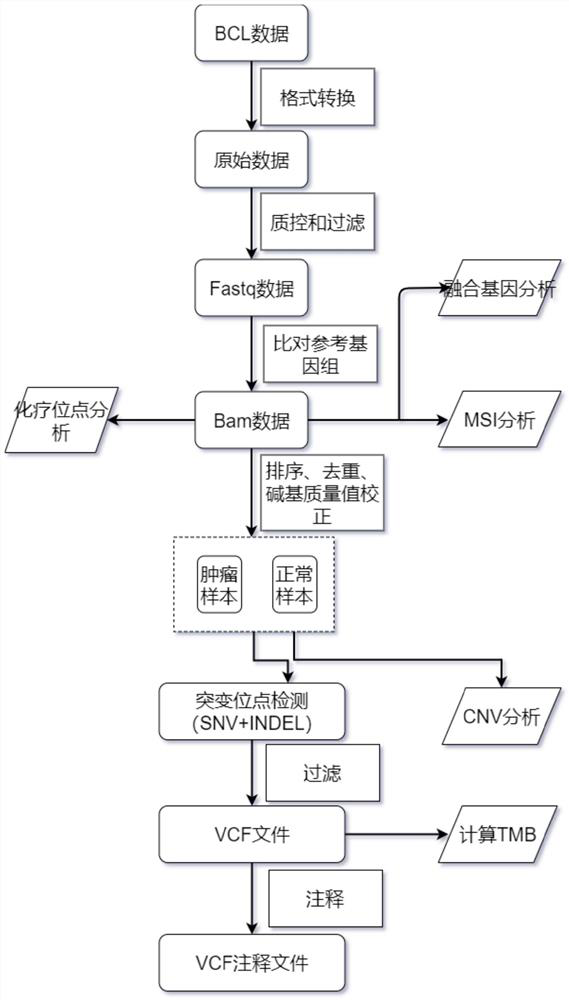
The invention relates to a gene panel used for detecting breast cancer gene mutation, and a detection method and application of the gene panel. The panel disclosed by the invention comprises 54419 pieces of targeted DNA (deoxyribonucleic acid) probes. The targeted DNA comprises the exon regions of 445 pieces of genes on the human genomes, 2573 pieces of MSI (microsatellite instability) sites and 566 position intervals used for detecting gene fusion. The detection method in the invention can be used for detecting SNV (single nucleotide variation), Indel (Insertion and Deletion), CNV (Copy Number Variation), Fusion, MSI, TMB (Tumor Mutational Burden), HLA (human leucocyte antigen) parting and the like of a tumor somatic cell multi-site DNA mutation. An optimal individual treatment medicine and scheme can be conveniently selected according to the genome features of patients in a breast cancer immunotherapy process.
Owner:北斗生命科学(广州)有限公司 +1
Gene expression classifier capable of predicting lung cancer patient prognosis and construction method of gene expression classifier
InactiveCN107292127ADemonstrating functional diversityStrong predictive powerBiostatisticsHybridisationAdjuvant therapyPhases of clinical research
The invention discloses a construction method of a gene expression classifier capable of predicting lung cancer patient prognosis. The method comprises a data training stage and a verification stage, wherein the training stage comprises a first stage and a second stage; at the first stage, a supervised machine learning method is used to establish a gene expression classifier prototype capable of predicting the lung cancer patient prognosis; and at the second stage, the machine learning method is further used to obtain the gene expression classifier capable of predicting the lung cancer patient prognosis. According to the method, the supervised machine learning method is used to obtain the gene expression classifier, and the prognosis of non-small cell lung cancer patients can be precisely predicted. The gene expression classifier has very high clinical transformation value. By performing gene expression detection of a gene panel, the non-small cell lung cancer patient with a high-risk gene risk score should receive adjuvant therapy, and the non-small cell lung cancer patient with a low-risk gene risk score should receive a low dose or be exempted from adjuvant therapy.
Owner:南京明捷生物医药检测有限公司
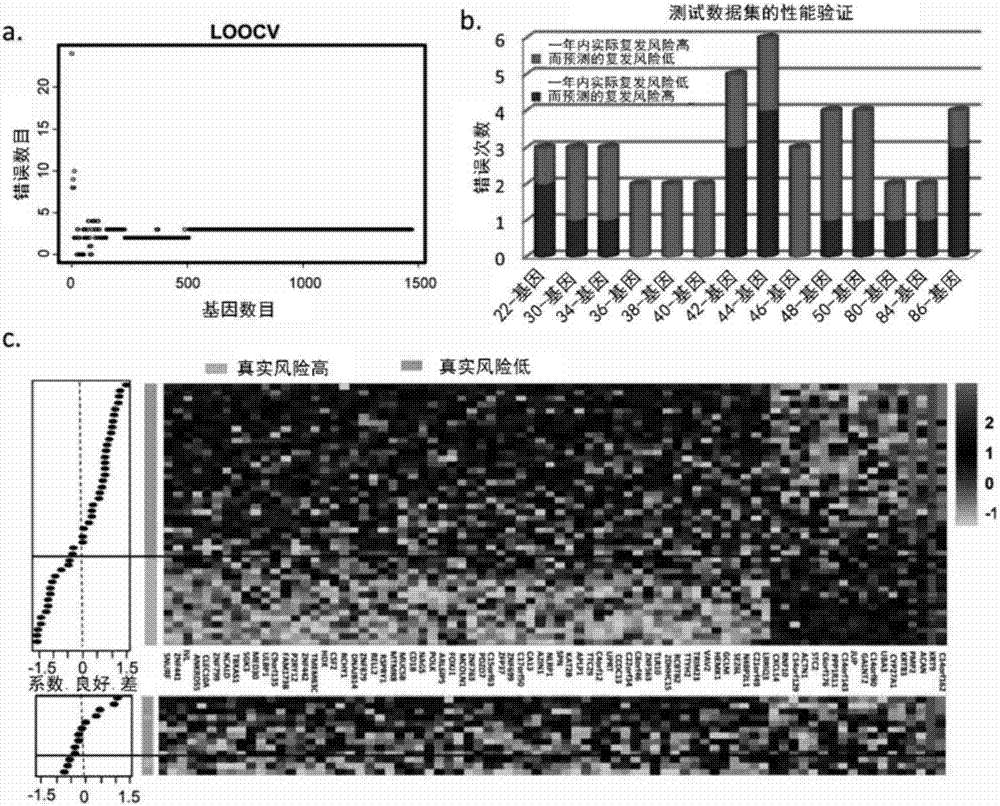
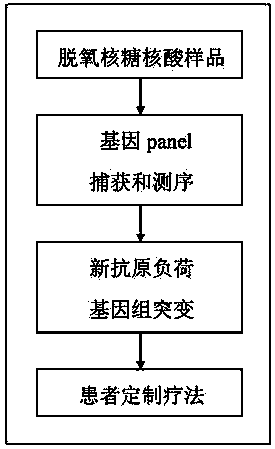
Gene panel for predicting new antigen load and detecting genome mutation
PendingCN110592213AQuick forecastEfficient forecastingMicrobiological testing/measurementDNA/RNA fragmentationAntigenIndividualized treatment
The invention relates to the fields of biotechnology, DNA mutation detection technology and bioinformatics, in particular to a gene panel for predicting a new antigen load and detecting genome mutation. Specifically, the invention relates to the gene panel which combines 839 genes and integrates 511 gene fusion events. The gene panel provided by the invention can rapidly, efficiently and accurately predict a new antigen load, and can also detect DNA mutation of multiple sites at one time, so that an optimal individualized treatment drug and scheme can be selected according to genomic characteristics of a patient in a cancer immunotherapy process. In addition, the gene panel provided by the invention can also be used for measuring various types of biomarkers such as SNV, Indel, CNV, gene fusion, MSI, TMB and HLA.
Owner:深圳市新合生物医疗科技有限公司
Peripheral blood gene markers for early diagnosis of parkinson's disease
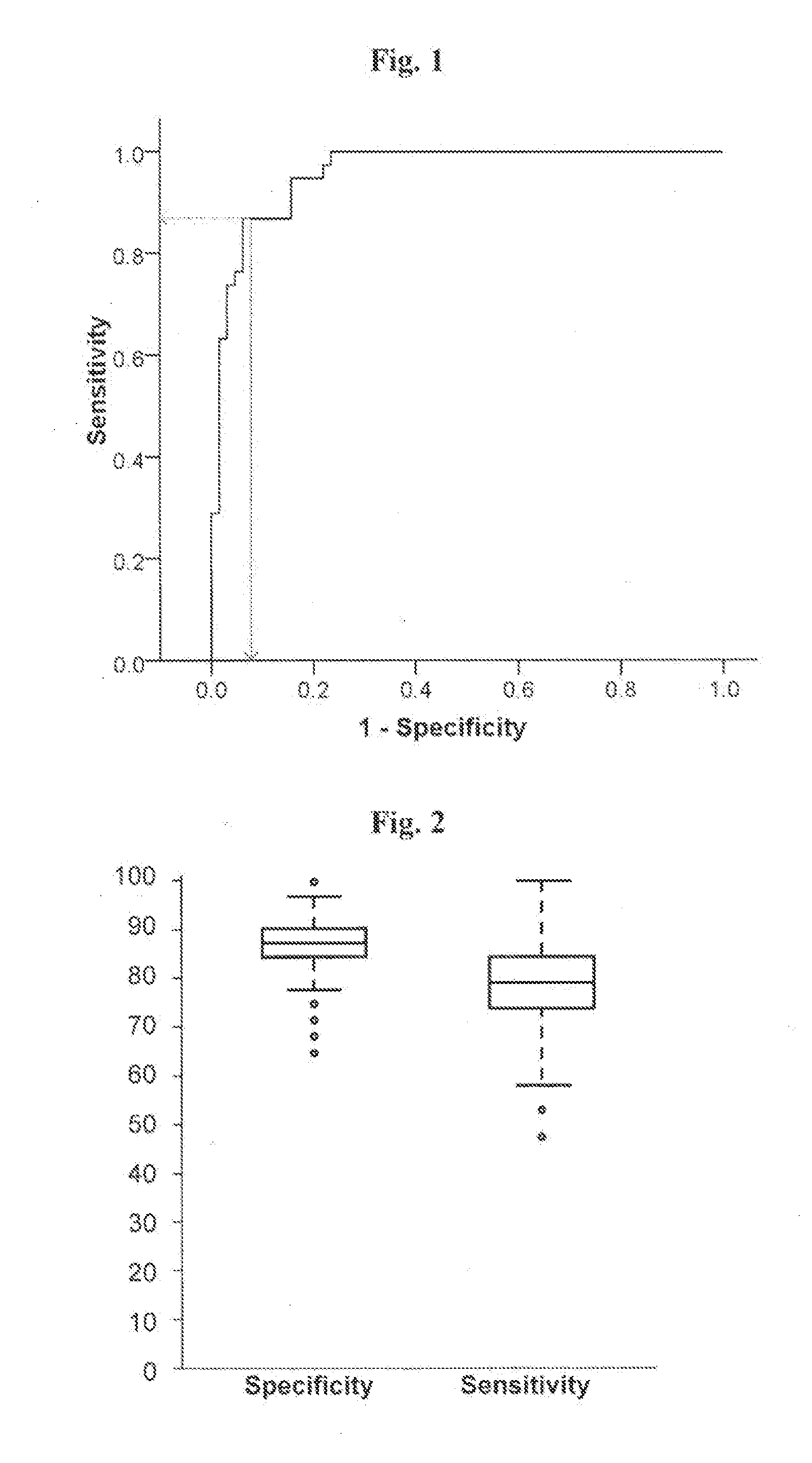
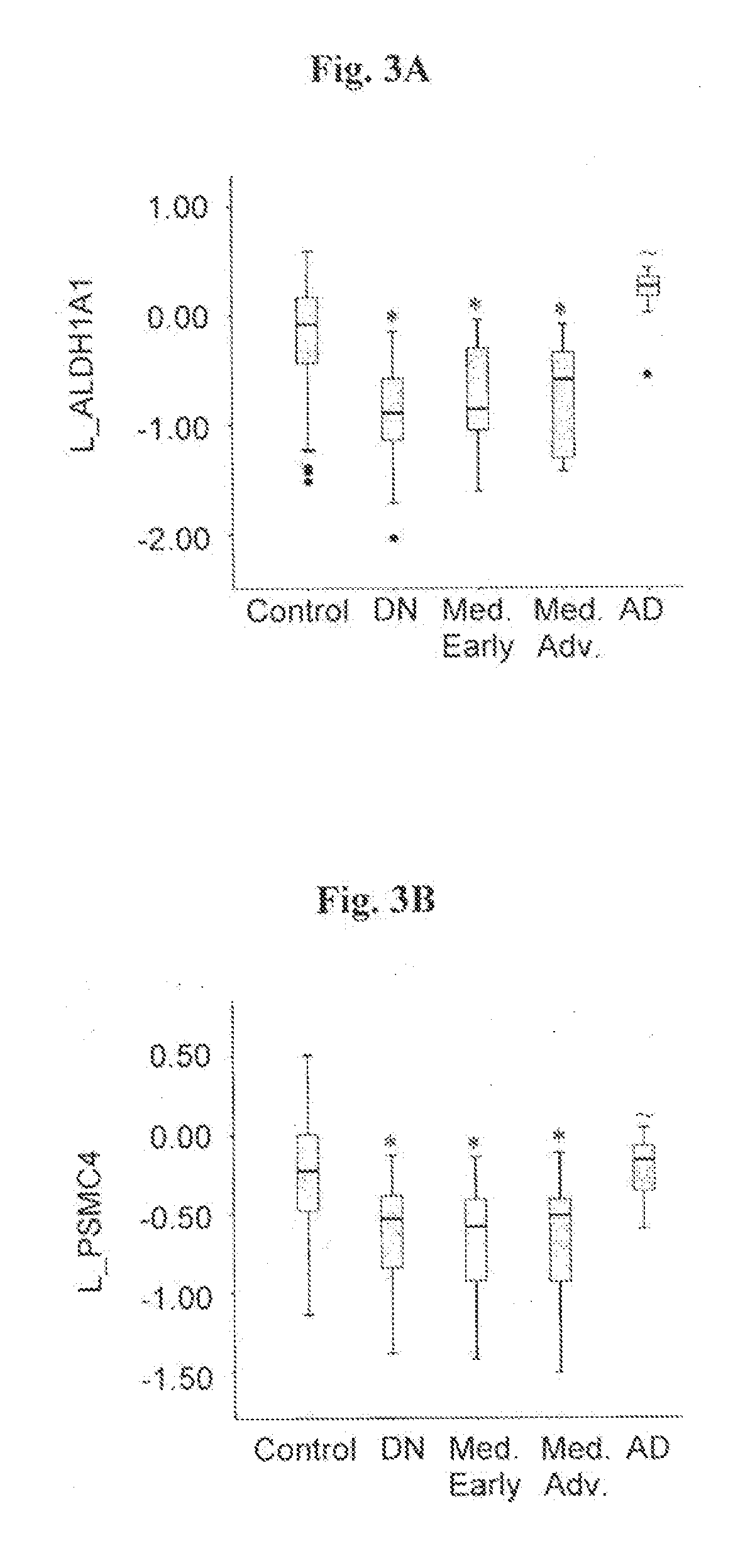
InactiveUS20130217028A1Increase and decrease levelMicrobiological testing/measurementBiological testingPhysiologyALDH1A1
The present invention relates to the use of molecular risk marker profiles for diagnosis of Parkinson's disease. More particularly, the invention provides methods for diagnosis of Parkinson's disease in an individual, utilizing certain profiles established based on the expression levels of certain genes, which together form a gene panel, in the peripheral blood of said individual, as well as kits for carrying out these methods. The profile encompass ALDH1A1.
Owner:MANDEL SILVA A +5
Markers and Methods for Assessing and Treating Ulcerative Colitis and Related Disorders Using 66 Gene Panel
ActiveUS20090054253A1Inhibit productionSugar derivativesMicrobiological testing/measurementUlcerative colitisMedicine
A method for prognostic or diagnostic assessment of a gastrointestinal-related disorder, such as ulcerative colitis, in a subject correlates the presence, absence, and / or magnitude of a gene in a sample with a reference standard to determine the presence and / or severity of the disorder, and / or the response to treatment for the disorder. The method enables identification of the effectiveness of candidate therapies.
Owner:CENTOCOR ORTHO BIOTECH
Markers for ovarian cancer and the uses thereof
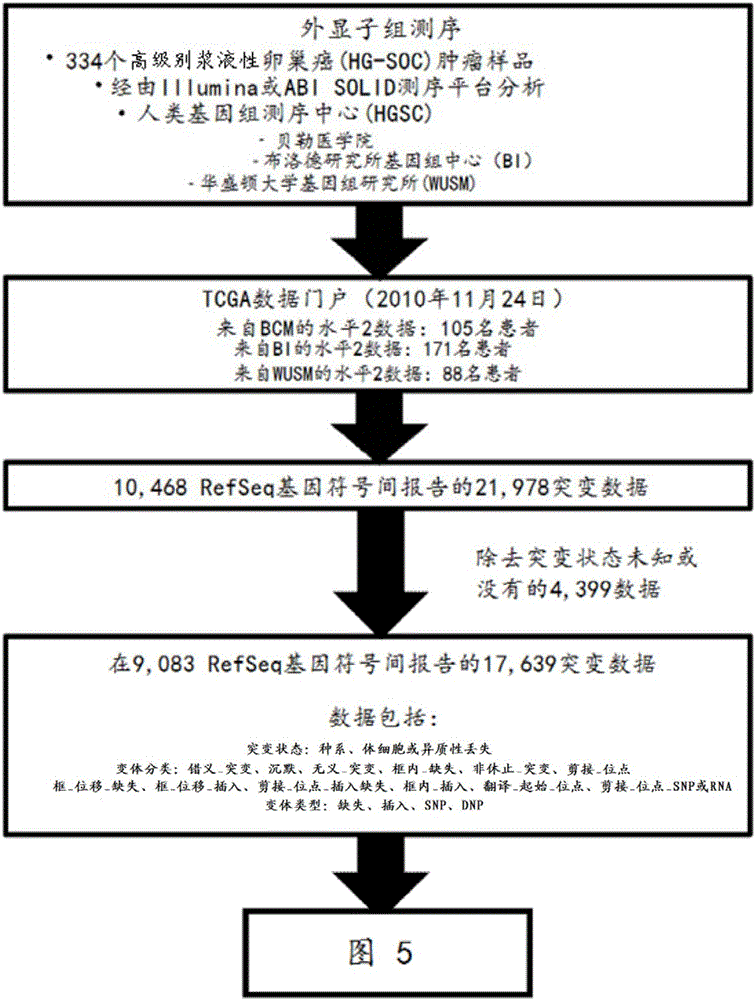
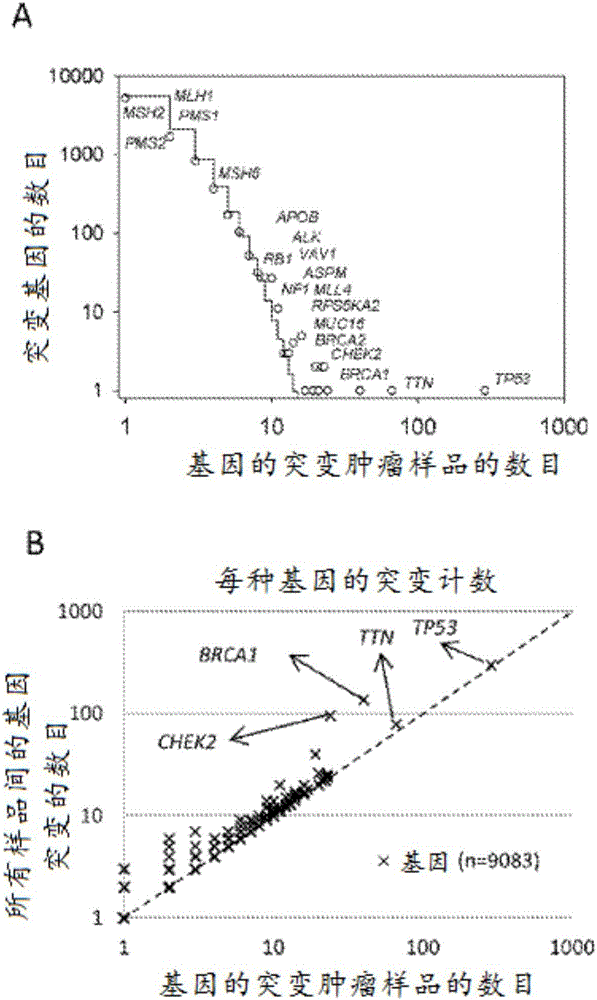
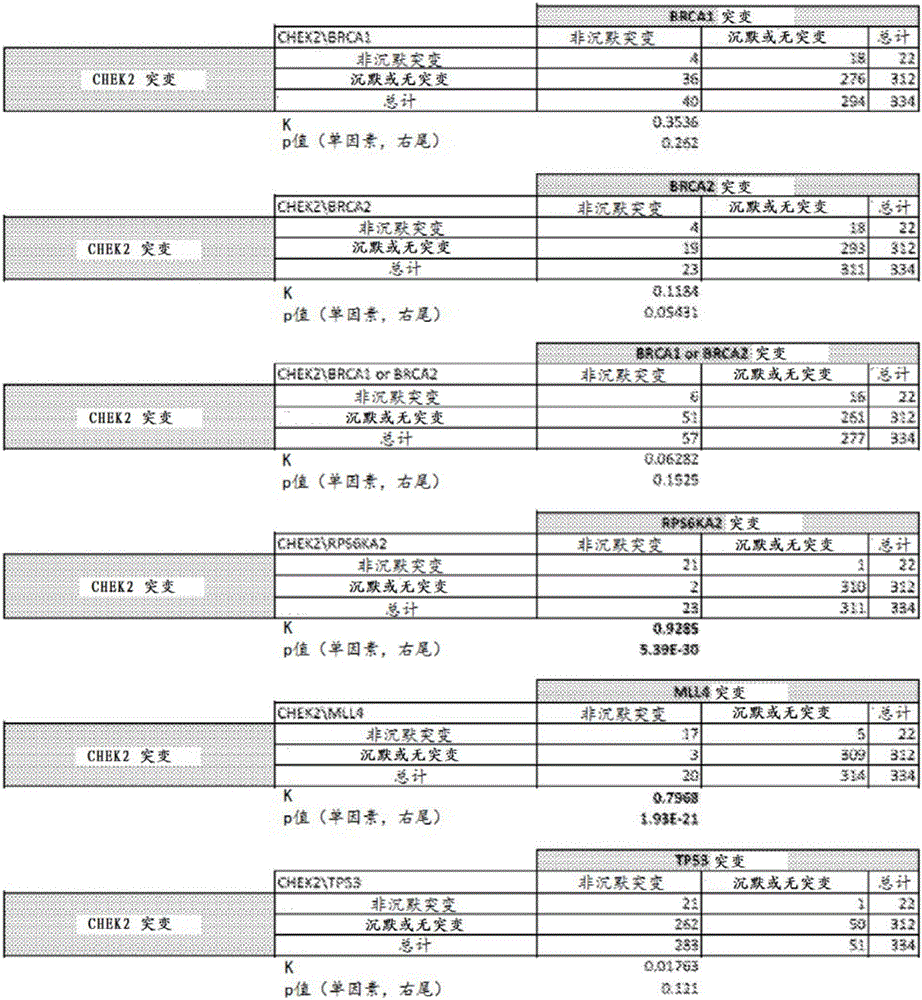
The present invention relates to markers for high-grade serous ovarian cancer (HG-SOC) and methods and uses thereof for diagnosing high-grade serous ovarian cancer (HG-SOC) and / or determining the prognosis of a subject suffering from high-grade serous ovarian cancer (HG-SOC) by determining the presence or absence of a mutation in a CHEK2 marker or mutations of markers from a 21 -gene panel comprising ADAMTSL3, ATR, CHEK2, ENAH, ERN2, GLI2, GYPB, KIAA1324L, LRRN2, MAP3K6, MAPK15, MET, MLL4, NIPBL, PCDH15, PPP1CC, PTCH1, PTK2B, RPS6KA2, RSU1 and TNC. It also relates to the use of markers CHEK2, RPS6KA2 and MLL4 in predicting the risk of developing high-grade ovarian serous ovarian cancer by determining germline mutations in at least one of these three markers.
Owner:AGENCY FOR SCI TECH & RES
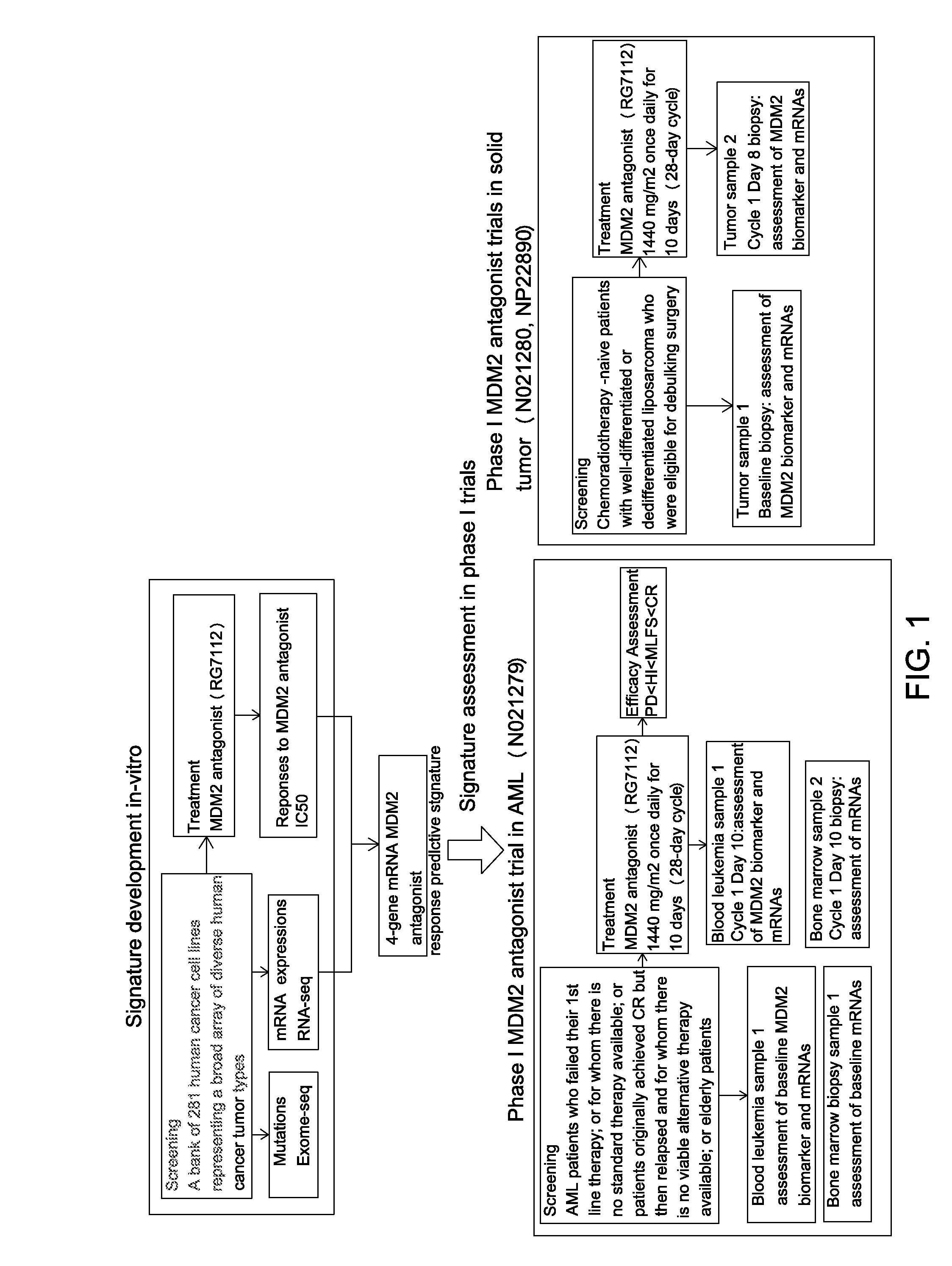
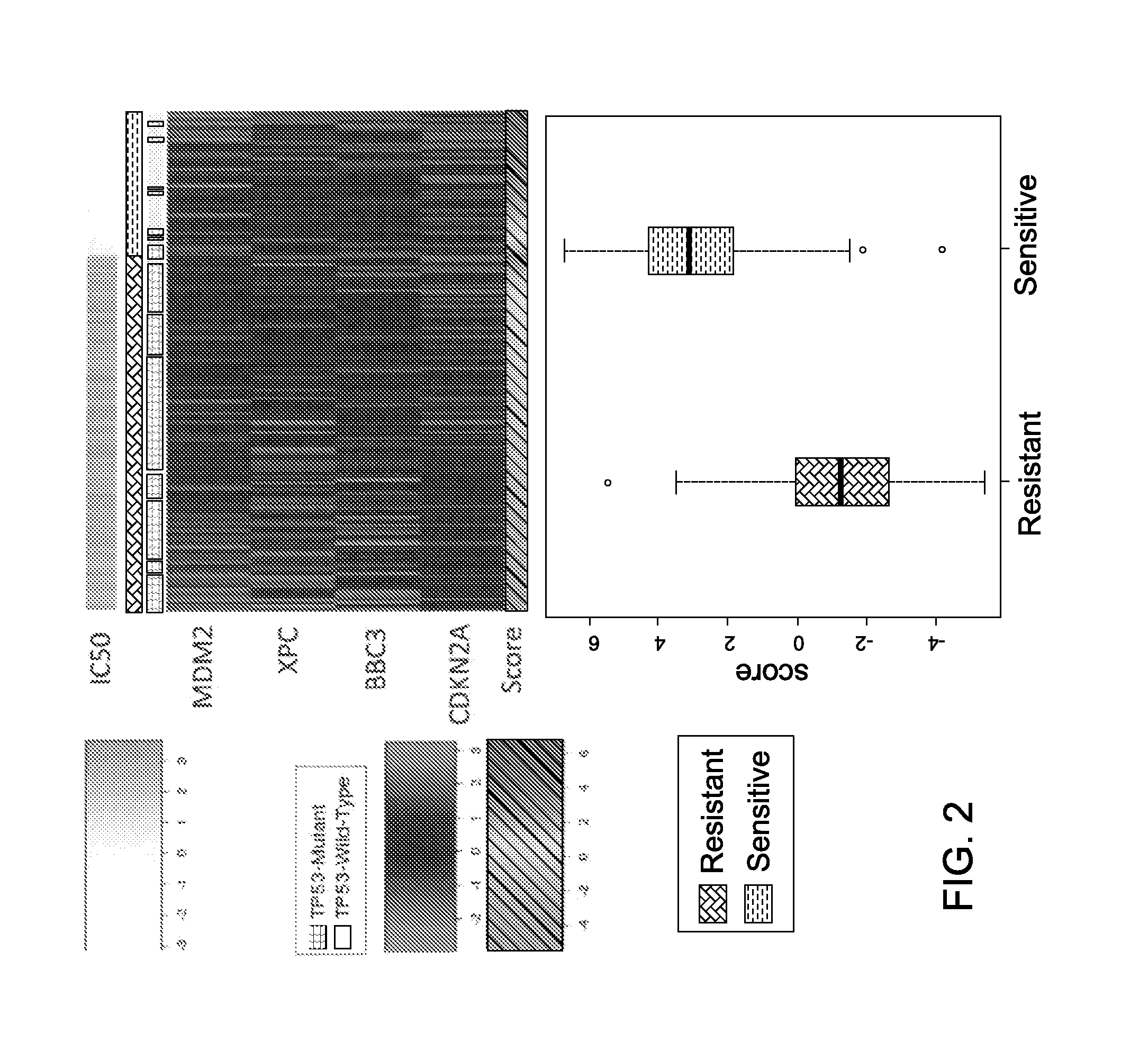
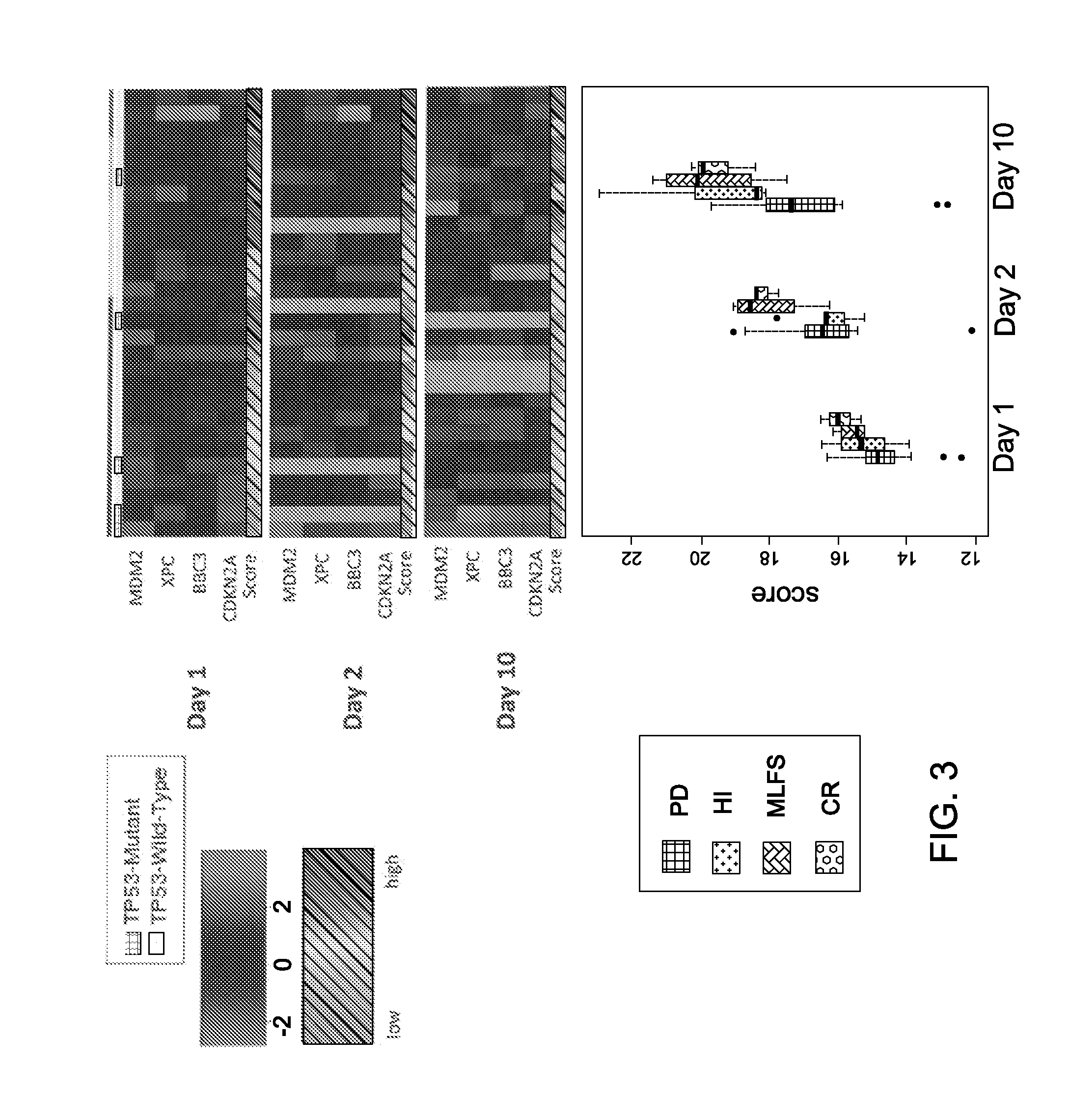
Mrna-based gene expression for personalizing patient cancer therapy with an MDM2 antagonist
Use of at least an MDM2 gene panel, preferably a four gene MDM2 gene panel, as a biomarker for predicting the response to a MDM2 antagonist.
Owner:F HOFFMANN LA ROCHE & CO AG
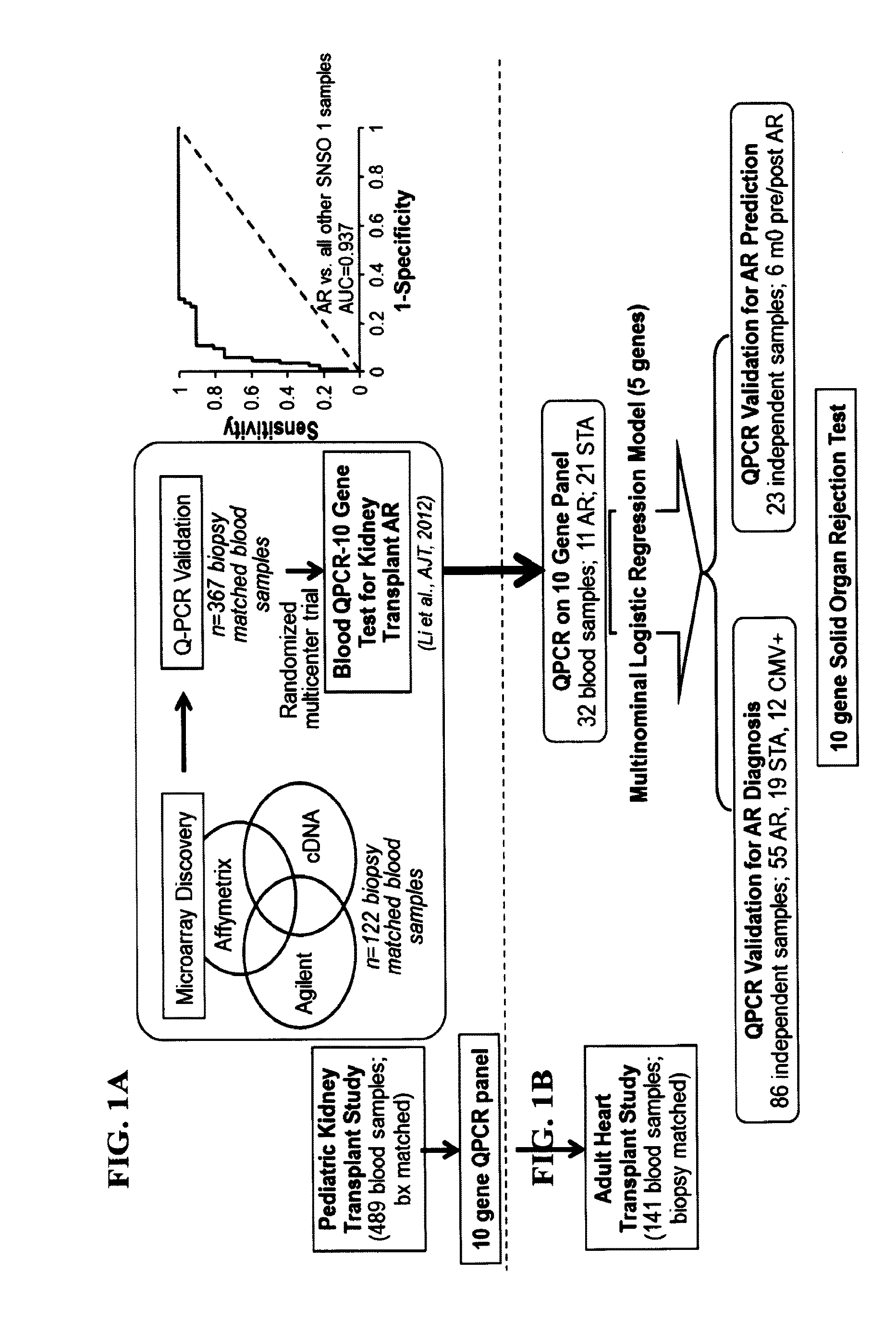
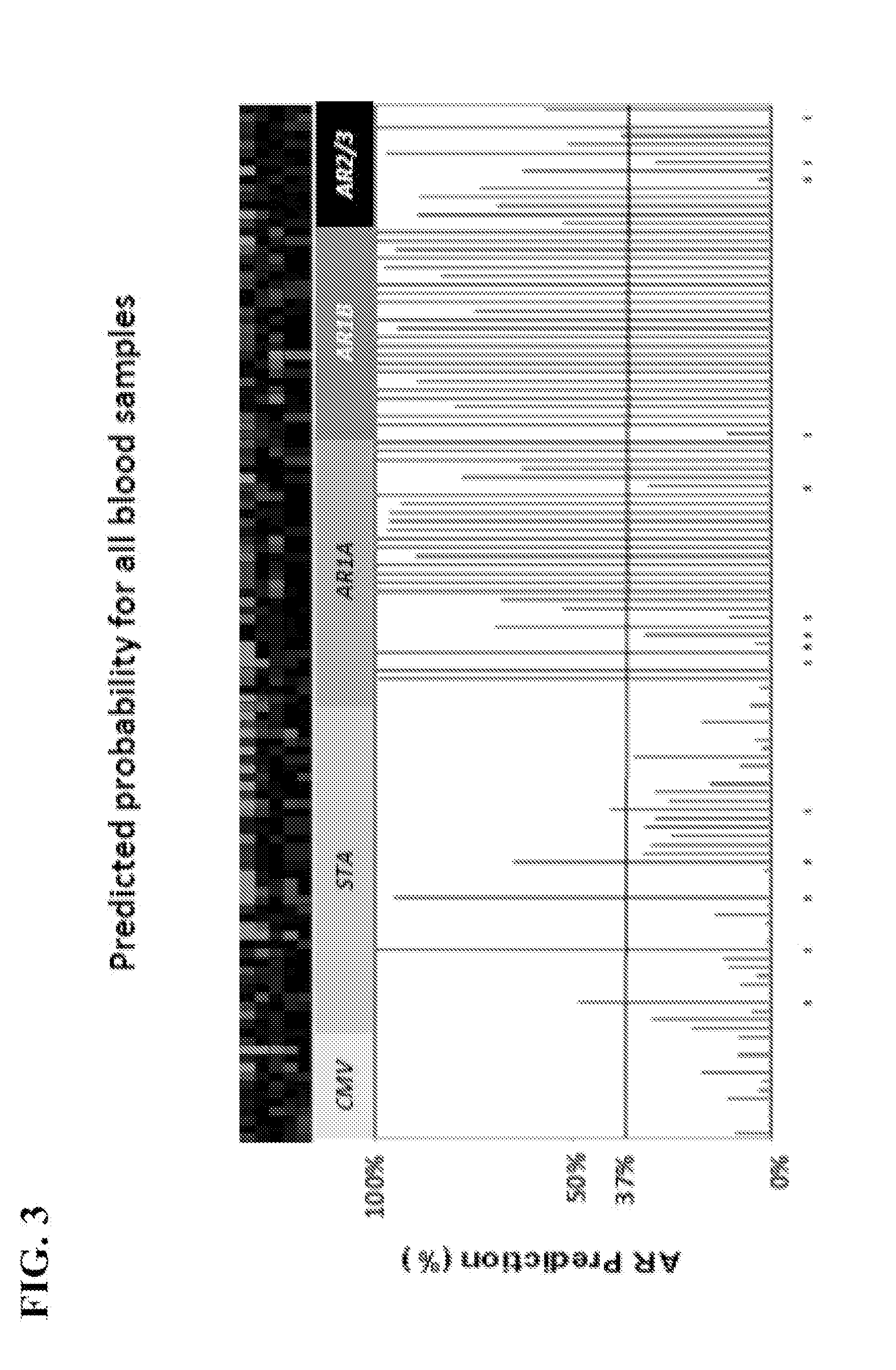
Compositions and methods for diagnosis and prediction of solid organ graft rejection
Owner:IMMUCOR GTI DIAGNOSTICS
Systems and methods for clonal replication and amplification of nucleic acid molecules for genomic and therapeutic applications
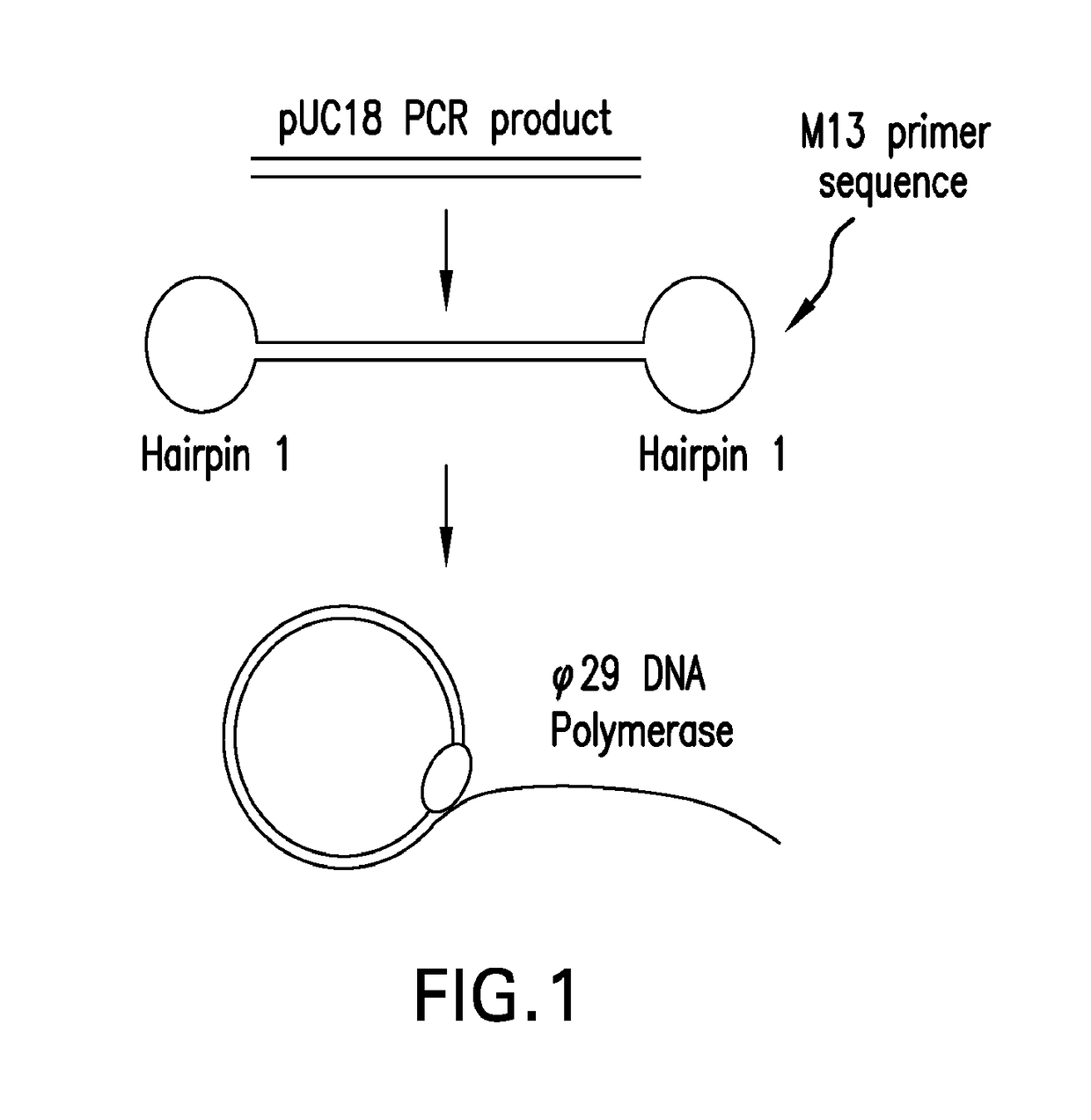
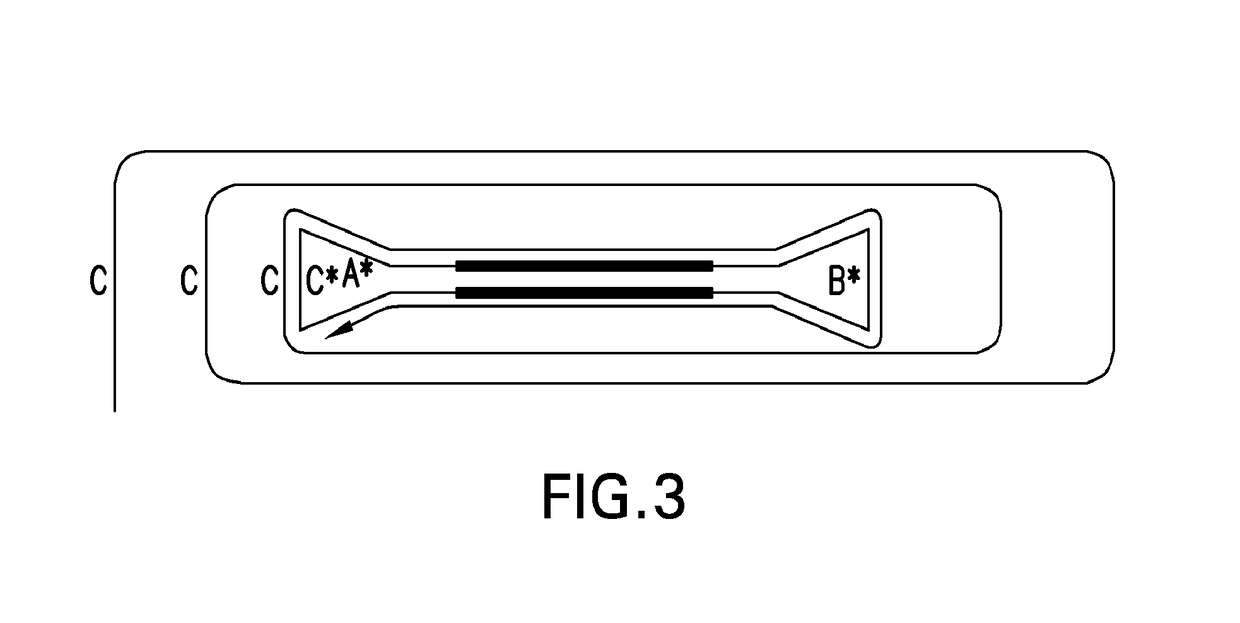
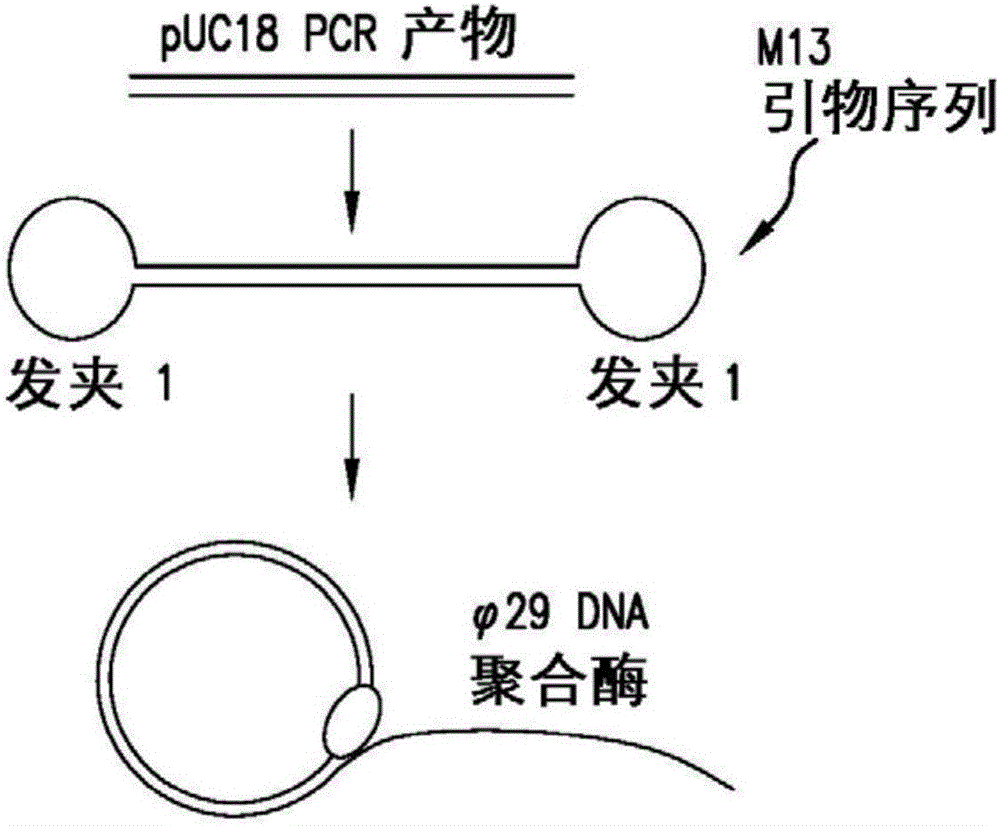
PendingUS20170067097A1Efficient productionCreate efficientlyMicrobiological testing/measurementChromosomal regionHaplotype
The present invention provides for methods, reagents, apparatuses, and systems for the replication or amplification of nucleic acid molecules from biological samples. In one embodiment of the invention, the nucleic molecules are isolated from the sample, and subjected to fragmenting and joining using ligating agents of one or more hairpin structures to each end of the fragmented nucleic molecules to form one or more dumbbell templates. The one or more dumbbell templates are contacted with at least one substantially complementary primer attached to a substrate, and subjected to rolling circle replication or rolling circle amplification. The resulting replicated dumbbell templates or amplified dumbbell templates are used in numerous genomic applications, including whole genome de novo sequencing; sequence variant detection, structural variant detection, determining the phase of molecular haplotypes, molecular counting for aneuploidy detection; targeted sequencing of gene panels, whole exome, or chromosomal regions for sequence variant detection, structural variant detection, determining the phase of molecular haplotypes and / or molecular counting for aneuploidy detection; study of nucleic acid-nucleic acid binding interactions, nucleic acid-protein binding interactions, and nucleic acid molecule expression arrays; and testing of the effects of small molecule inhibitors or activators or nucleic acid therapeutics.
Owner:REDVAULT BIOSCI
Systems and methods for clonal replication and amplification of nucleic acid molecules for genomic and therapeutic applications
The present invention provides for methods, reagents, apparatuses, and systems for the replication or amplification of nucleic acid molecules from biological samples. In one embodiment of the invention, the nucleic molecules are isolated from the sample, and subjected to fragmenting and joining using ligating agents of one or more hairpin structures to each end of the fragmented nucleic molecules to form one or more dumbbell templates. The one or more dumbbell templates are contacted with at least one substantially complementary primer attached to a substrate, and subjected to rolling circle replication or rolling circle amplification. The resulting replicated dumbbell templates or amplified dumbbell templates are used in numerous genomic applications, including whole genome de novo sequencing; sequence variant detection, structural variant detection, determining the phase of molecular haplotypes, molecular counting for aneuploidy detection; targeted sequencing of gene panels, whole exome, or chromosomal regions for sequence variant detection, structural variant detection, determining the phase of molecular haplotypes and / or molecular counting for aneuploidy detection; study of nucleic acid - nucleic acid binding interactions, nucleic acid - protein binding interactions, and nucleic acid molecule expression arrays; and testing of the effects of small molecule inhibitors or activators or nucleic acid therapeutics.
Owner:REDVAULT BIOSCI
Markers and methods for assessing and treating crohn's disease and related disorders
ActiveUS20090156418A1Microbiological testing/measurementLibrary screeningMolecular Targeted TherapiesTargeted therapy
A method for assessment of the suitability of and / or effectiveness of a target therapy for a gastrointestinal-related disorder, such as Crohn's disease, in a subject evaluates the presence, absence, and / or magnitude of expression of one or more genes in a 10-member gene panel in a sample. The method enables identification of the effectiveness of target therapies prior to or after starting a patient on such therapies.
Owner:JANSSEN BIOTECH INC
Pulmonary thromboembolism gene panel kit and application thereof
PendingCN109652525AIncreased sequencing depthIncrease coverageMicrobiological testing/measurementTarget captureMulti aspect
The invention relates to the technical field of biomedicine and particularly provides a liquid phase gene panel capture kit for detecting genes related to pulmonary thromboembolism and application thereof. The liquid phase gene panel capture kit comprises a capture probe covering 92 genes. The design of the gene panel capture kit integrates multi-level and multi-aspect genetic factors of pulmonaryembolism, is comprehensive in coverage and has obvious pertinence in target capture, genetic detection can be simultaneously conducted on genes with large fluxes, sequencing depth and coverage degreeof the genes can be effectively improved by efficiently enriching DNA of exon areas of target genes of people and adopting subsequent sequencing, and guarantee is provided for obtaining of more accurate gene genetic information and results.
Owner:SHENZHEN PEOPLES HOSPITAL
Markers and methods for assessing and treating Crohn's and related disorders
ActiveUS8557745B2Microbiological testing/measurementLibrary screeningMolecular Targeted TherapiesGenome
A method for assessment of the suitability of and / or effectiveness of a target therapy for a gastrointestinal-related disorder, such as Crohn's disease, in a subject evaluates the presence, absence, and / or magnitude of expression of one or more genes in a 10-member gene panel in a sample. The method enables identification of the effectiveness of target therapies prior to or after starting a patient on such therapies.
Owner:JANSSEN BIOTECH INC
Classification of tumor microenvironments
PendingUS20210174908A1Reduce the burden onImprove survival rateOrganic active ingredientsPeptide/protein ingredientsBiologic markerExpression gene
The disclosure provides population and non-population-based classifiers to categorize patients and cancers. The population-based classifiers disclosed integrate signatures, i.e., global scores related to the expression of genes in particular gene panels. The non-population-based classifiers are generated using machine-learning techniques (e.g., regression, random forests, or ANN). Each type of classifier stratifies patients and cancers according to tumor microenvironments (TME) as biomarker-positive or biomarker-negative, and treatment decisions are then guided by the presence / absence of a particular TME. Also provided are methods for treating a subject, e.g., a human subject, afflicted with cancer comprising administering a particular therapy depending on the classification of the cancer's TME according to the disclosed classifiers. Also provided are personalized treatments that can be administered to a subject having a cancer classified into a particular TME, and gene panels that can be used for identifying a human subject afflicted with a cancer suitable for treatment with a particular therapeutic agent.
Owner:ONCXERNA THERAPEUTICS INC
Gene panel for detecting single-gene genetic hypertension and application of gene panel
InactiveCN109750101AThe detection process is fastAchieve early detectionBioreactor/fermenter combinationsBiological substance pretreatmentsDetection rateDisease cause
The invention discloses a gene panel for detecting single-gene genetic hypertension and application of a gene panel. By means of the gene panel, potential pathogenic gene mutation of single-gene genetic hypertension can be screened out of massive genetic information by means of targeted high-throughput sequencing in combination of a bioinformatics analysis means, and the positive detection rate ofmutation sites of single-gene genetic hypertension and application can be remarkably increased. The detection result of the gene panel is combined with clinical phenotype of a patient, etiological diagnosis of single-gene genetic hypertension and application of gene panel can be effectively assisted, and therefore the clinical disease diagnosis rate is increased, a specific and effective intervention treatment scheme is implemented aiming to specific illness states, and the effects of early finding, early intervention and prognosis improvement on single-gene genetic hypertension are achieved.According to the technical scheme, the remarkable advantages of being high in gene detection speed, high in disease diagnosis accurate and the like are achieved.
Owner:FUWAI HOSPITAL CHINESE ACAD OF MEDICAL SCI & PEKING UNION MEDICAL COLLEGE
Cancer gene detection kit based on second-generation sequencing probe capture method
PendingCN111662981AMultiple Medication GuidanceImprove uniformityMicrobiological testing/measurementSingle strandTargeting chemotherapy
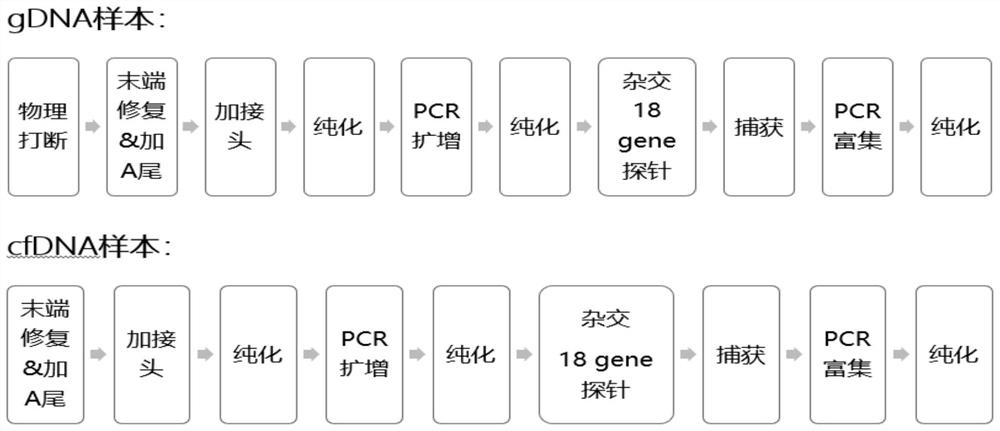
The invention discloses a cancer gene detection kit based on a second-generation sequencing probe capture method. The cancer gene detection kit comprises quality control materials, library building reagents and an RNA probe; quality control materials: a negative quality control material and a positive quality control material; library building reagents: a tail end repairing and A adding reagent, ajoint adding reagent, an amplification reagent, a hybridization reagent, a capture reagent and an amplification reagent; the RNA probe is a probe panel; the synthesis type of the RNA probe is an RNAsingle strand; the length interval is 90-120 nt; and biotin is marked. The cancer gene detection kit in the invention aims at patients with lung cancer, colorectal cancer, breast cancer and ovarian cancer. Detection of 18 genes panel is carried out; the receptivity of medicines, such as targeting and chemotherapy, on a patient is comprehensively interpreted from the gene level; according to the difference of individual gene levels, an accurate personalized treatment scheme is made; immune medication treatment is guided; the sensitivity and toxic and side effects of chemotherapy drugs are predicted; a medication prognosis condition is dynamically monitored, etc.
Owner:俊兮生物科技(上海)有限公司
Stratification of Left-Side and Right-Side Colon Cancer
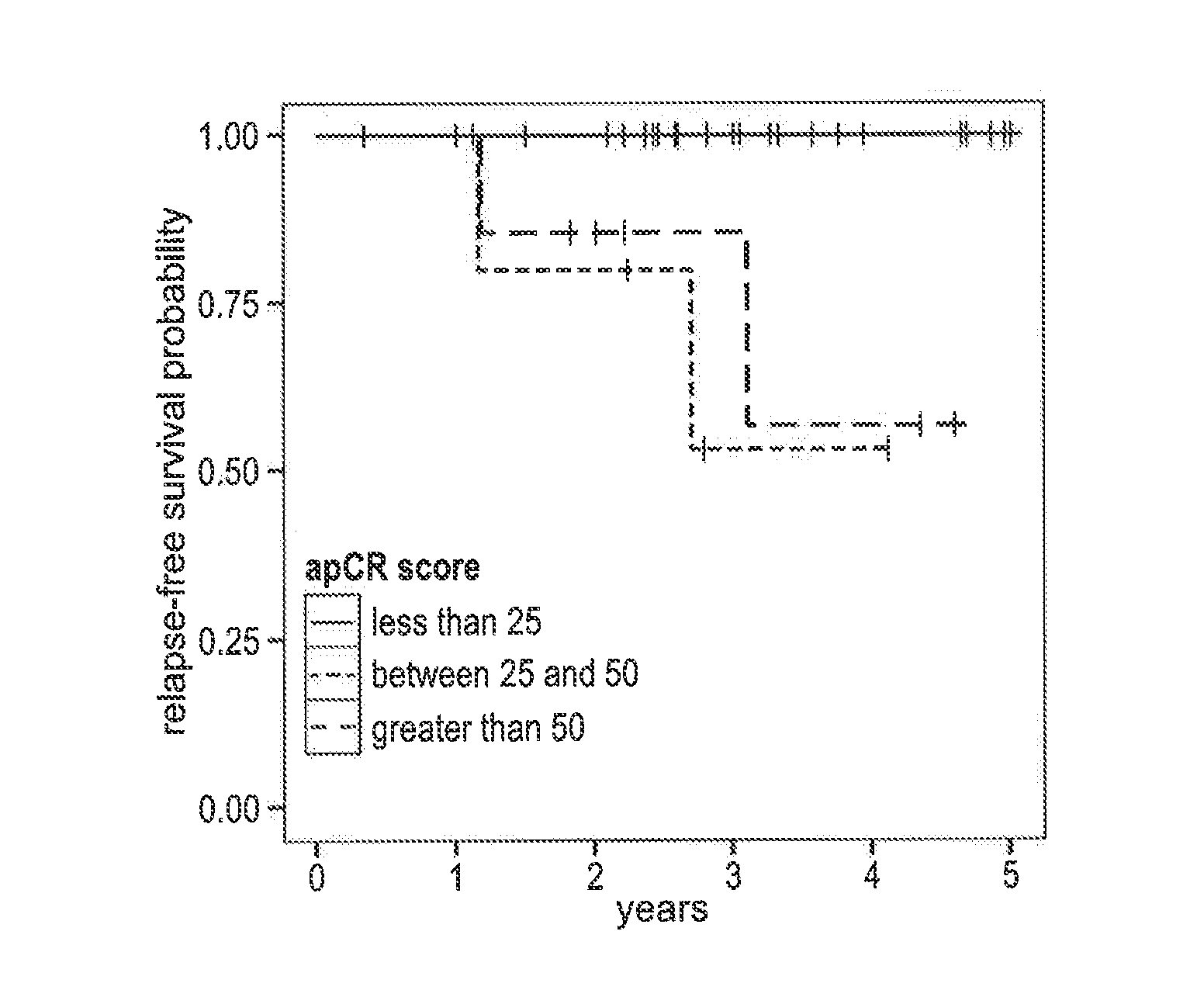
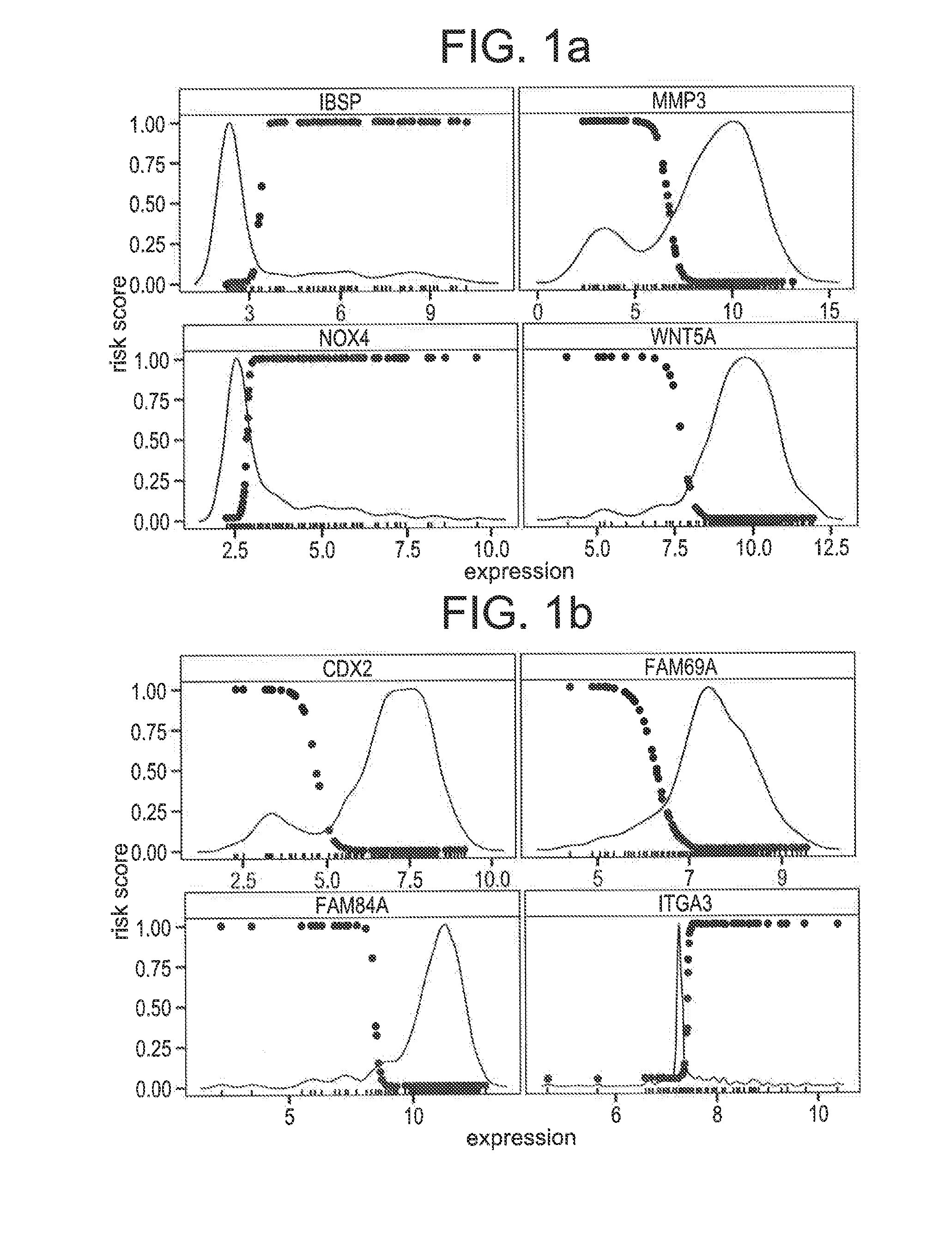
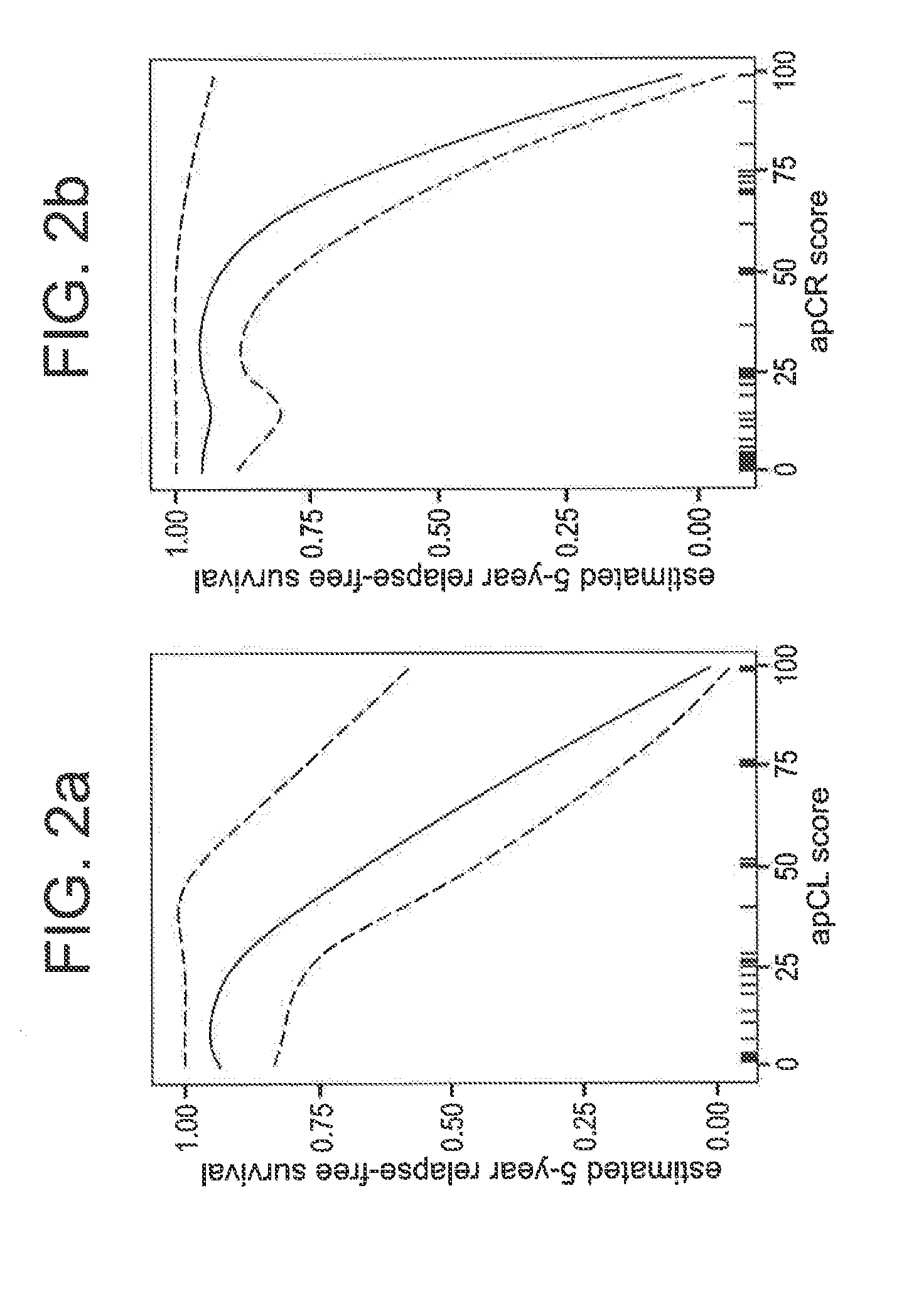
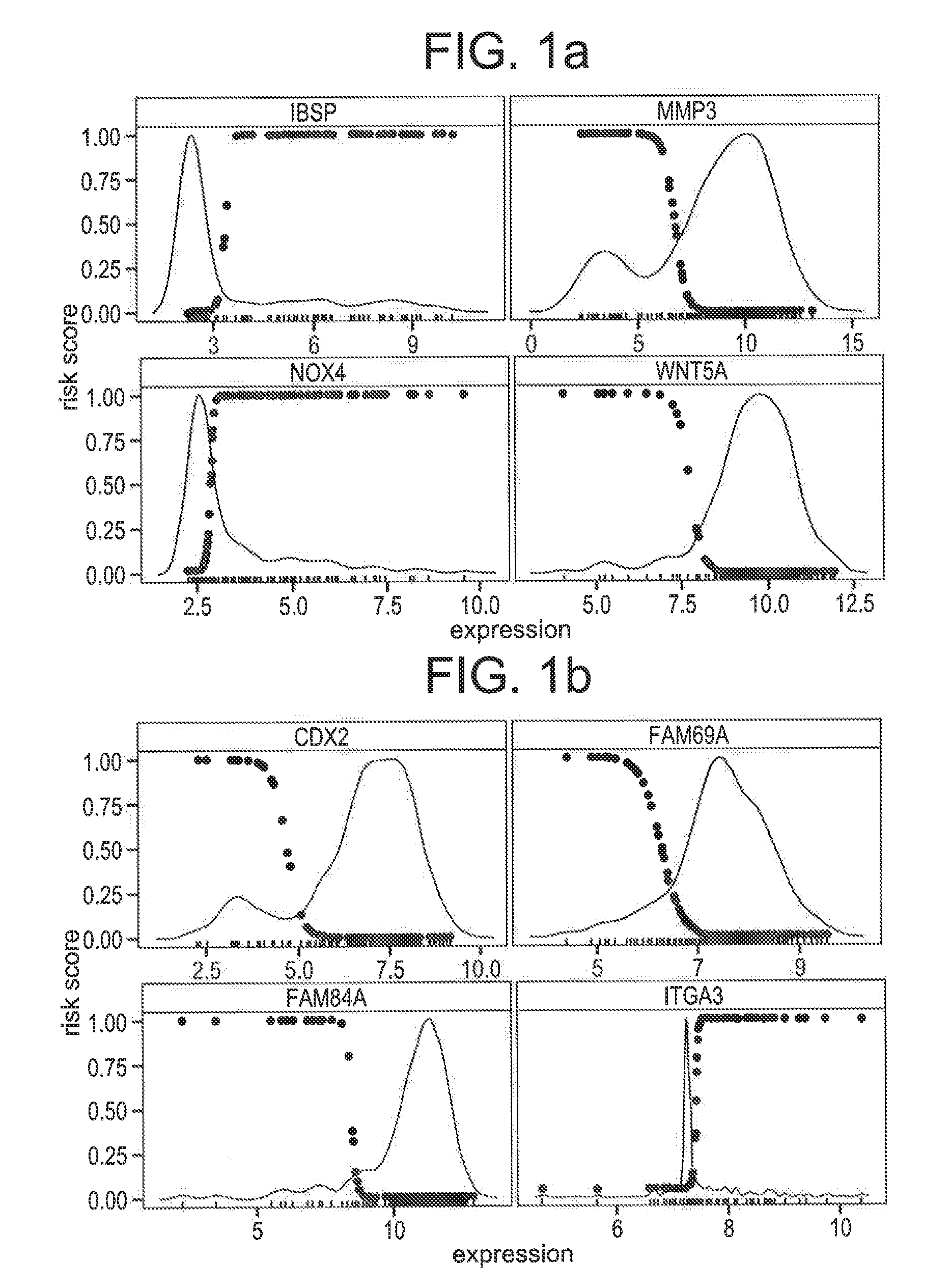
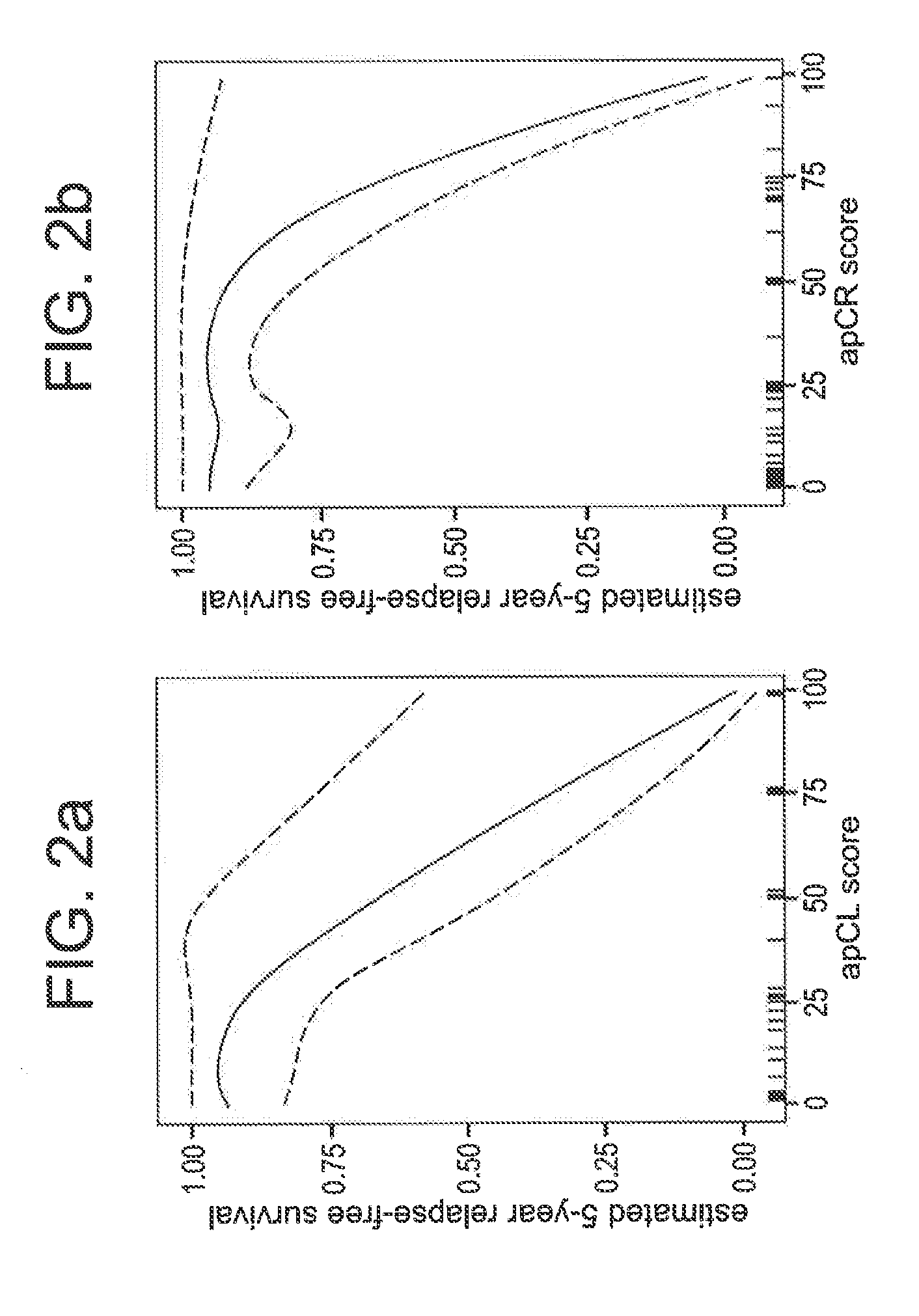
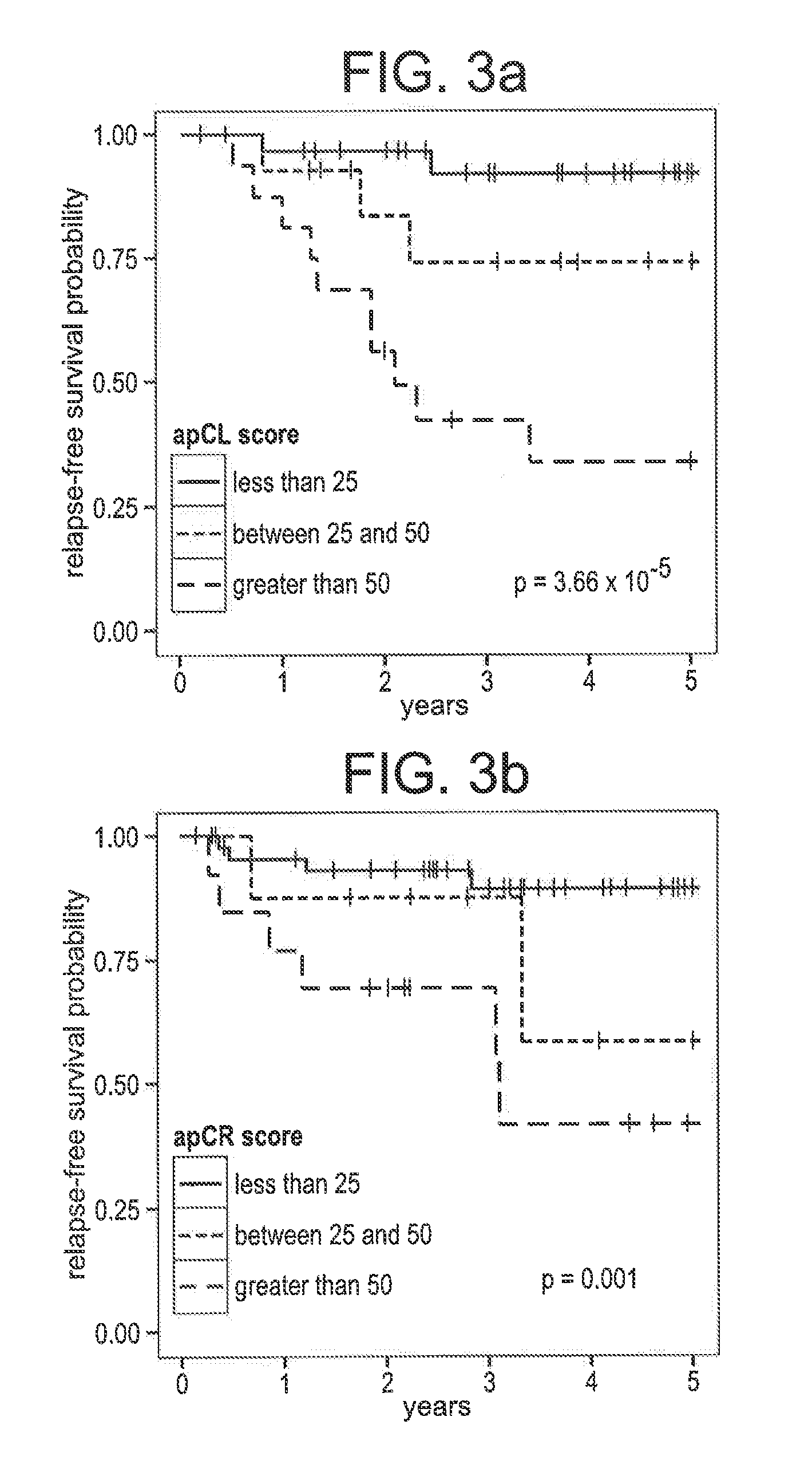
Compositions / methods for employing fresh-frozen or FFPE colon cancer tissue in left side colon cancer (LCC) and right-side colon cancer (RCC) disease patients for risk of relapse assessment / stratification is provided (3 strata and a 4 strata methodology). An RCC gene panel of 4 genes (FAM69A, CDX2, FAM84A, ITGA3), and 9 genes (FAM69A, CDX2, ITGA3, FAM84A, ITPRIP, RAB3B, SMAD3, PCSK5, MMP28), is provided. An LCC gene panel of 4 genes (NOX4, WNT5A, MMP3, IBSP), and a 9 genes (MMP3, WINT5A, NOX4, IBSP, SLC16A6, CYPIBI, TFAP2C, MATN3, ANKRD6), is provided. A microchip-based clinical tool, and a kit including a microchip, is presented. The invention also describes a computer-implemented method for assessing relative risk of relapse in LCC and / or RCC disease. An individual patient scoring method that presents a continuous stratification score useful in the post-surgical colon cancer management of LCC and / or RCC patient is also presented.
Owner:UNIV OF NOTRE DAME DU LAC
Gene combination for screening hereditary heart disease and application thereof
PendingCN110863045AUniversal screeningReduce testing costsMicrobiological testing/measurementTNNT2Heart disease disorder
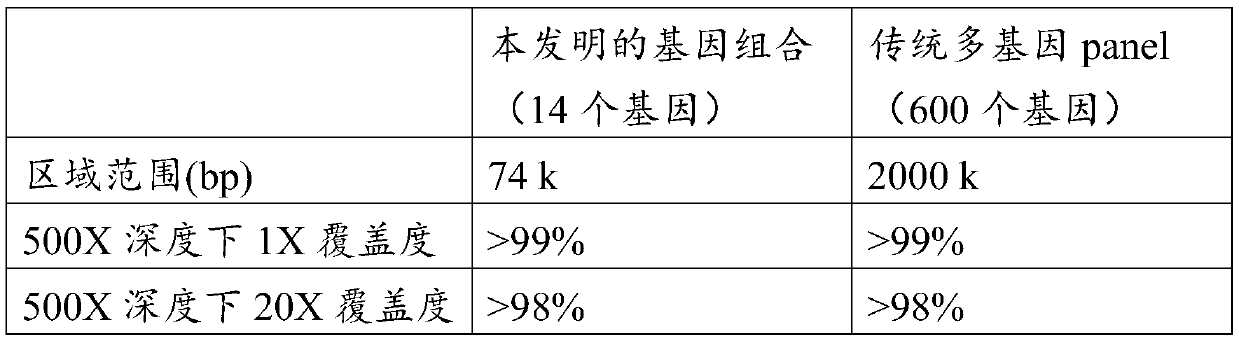
The present invention provides gene combinations for screening hereditary heart disease. The gene combination is composed of fourteen genes including MYH7, MYBPC3, TNNT2, TNNI3, LMNA, DSP, SCN5A, DSG2, PKP2, KCNH2, KCNQ1, RYR2, FBN1 and LDLR. The invention also provides application of the gene combination in preparation of a reagent for detecting hereditary heart disease. Compared with traditionalmulti-gene panel methods, the method for screening the hereditary heart disease by adopting the gene combination has the advantages that the number of detected genes and the detection cost are remarkably low, and the detection coverage and accuracy are not remarkably reduced. Based on high-throughput sequencing for screening hereditary heart diseases and preventing sudden death risks, the gene combination has the advantages of being low in cost and high in cost performance, the cost and detection efficiency are balanced, the gene combination is suitable for general screening of large-scale populations, and the low-cost detection technology can benefit most populations.
Owner:深圳瑞奥康晨生物科技有限公司
Gene panel kit of Immotile cilia syndrome and application of gene panel kit
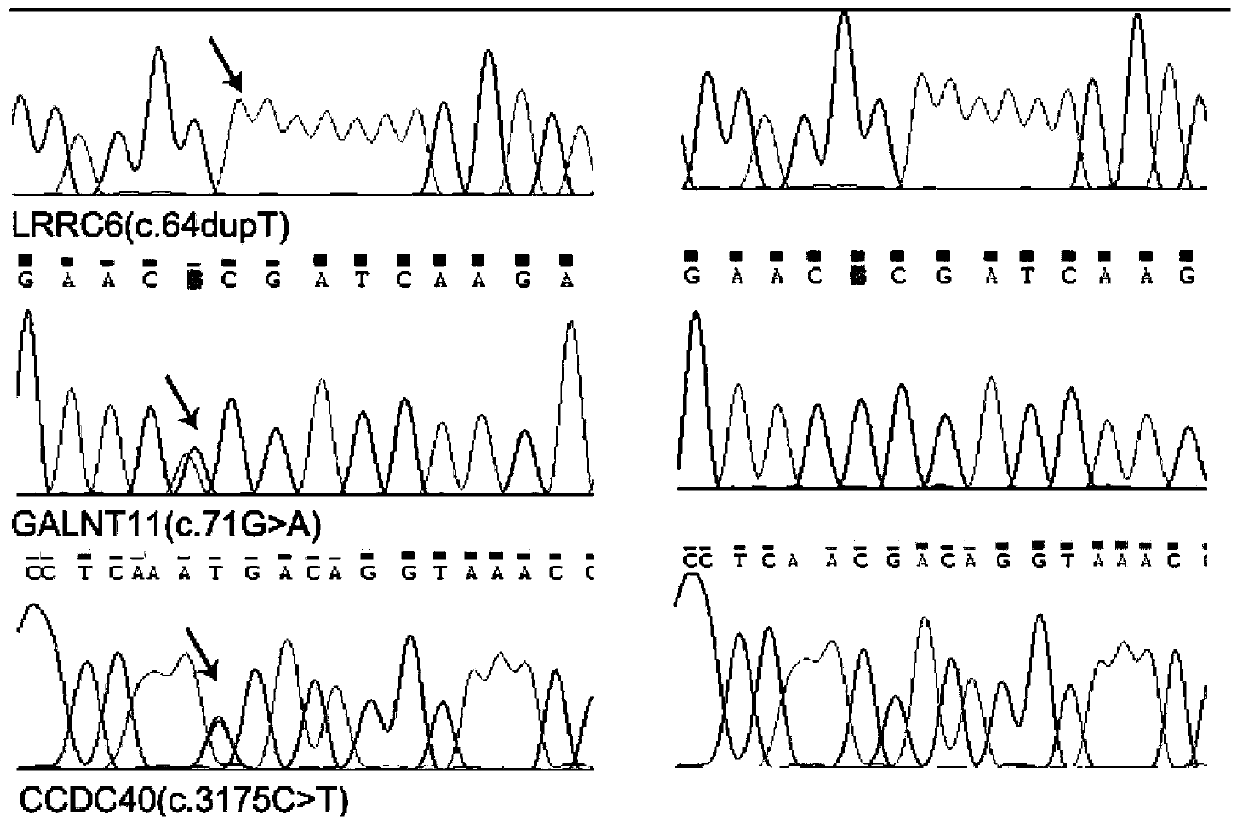
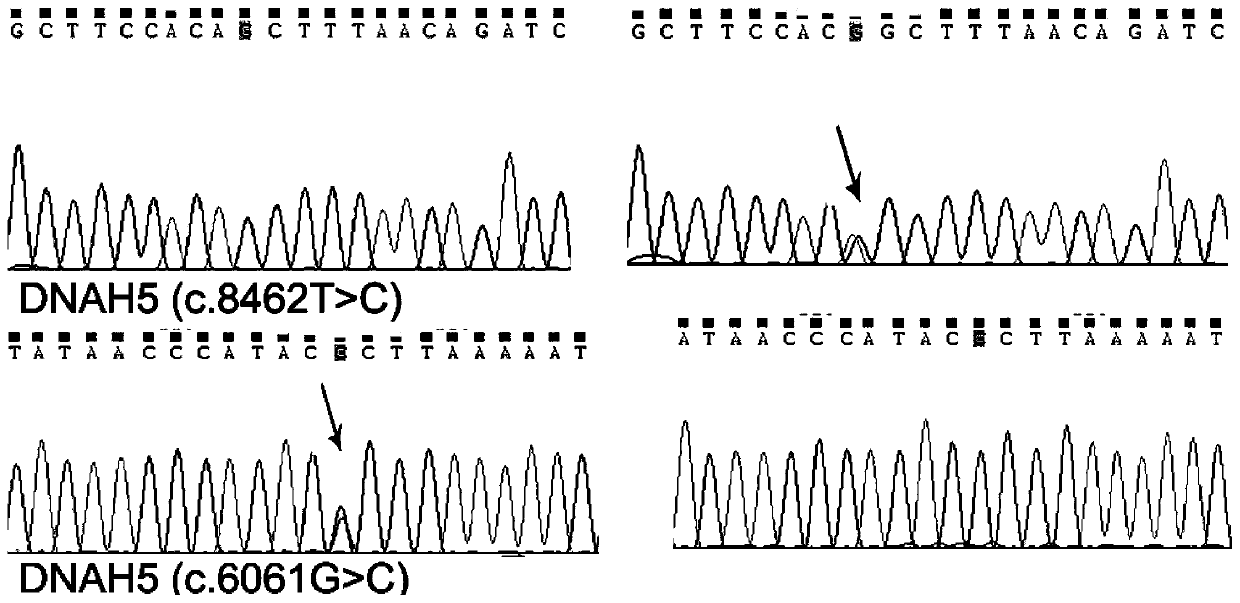
PendingCN110438220AAccurate detectionReduce birth defectsMicrobiological testing/measurementDeep sequencingGene panel
The invention relates to the field of biomedical technologies and disease diagnosis, and particularly discloses a gene panel capture kit for detecting genetic mutation of a pathogenic gene of Immotilecilia syndrome and application of the gene panel capture kit. The liquid phase capture kit of the gene panel comprises 49 probes corresponding to the related pathogenic gene of the Immotile cilia syndrome. The design of the gene panel capture kitsynthesizes the pathogenic factors of the Immotile cilia syndrome genetic defects of cilia immobility syndrome in multiple layers and aspects, comprehensive covering is achieved, the target capture of the gene panel capture kit has obvious pertinence, and the pathogenic gene with large flux can be accurately and simultaneously captured and genetic-detected. Human target gene exon region DNA is efficiently enriched, and then high-throughput and high-depth sequencing is conducted on a second generation sequencing platform, the depth and coverage ofgene sequencing can be effectively improved, and convenience is provided for obtaining more accurate genetic mutation information of gene pathogenicity and disease prevention and diagnosis.
Owner:SHENZHEN PEOPLES HOSPITAL
Gene panel for nervous system tumor detection, kit and application thereof
PendingCN113736878AAccurate detectionReduce the cost of genetic testingMicrobiological testing/measurementProteomicsGenes mutationNervous system
The invention belongs to the technical field of nervous system tumor multi-gene detection, and discloses a gene panel for detecting nervous system tumors, important exon regions and partial intron regions of 454 genes are enriched by using a biological probe hybridization method, and the detection and analysis contents comprise somatic mutation, embryonic mutation, copy number variation, fusion gene and other variation, which aims to achieve accurate detection. The multi-gene screening list is made into a probe, high-risk genes are detected in a targeted mode through hybridization capture sequencing, gene mutation with guiding significance on diagnosis and treatment can be detected more efficiently, the gene detection cost of patients and the operation difficulty of experimenters can be reduced, meanwhile, some tumor patients can be possibly helped to participate in clinical tests, and important guiding significance is provided for clinical diagnosis and discovery of target spots of targeted therapy.
Owner:FUDAN UNIV SHANGHAI CANCER CENT
Stratification of left-side and right-side colon cancer
Owner:UNIV OF NOTRE DAME DU LAC
Multimarker panel
InactiveUS20130303400A1Easy diagnosisNucleotide librariesMicrobiological testing/measurementProtein markersOncology
The invention is directed to a method of diagnosing a malignant ovarian tumor disease in a subject, which comprises —providing a sample of peripheral blood cells (PBC) of the subject, —measuring the expression of a multimarker gene panel comprising at least NEAT1, BC037918, C1 orf63, PRIC285, OSM, and optionally further genes or protein markers, and —comparing to a reference value, the differential expression being indicative of a malignant ovarian tumor, and a set of reagents to determine the expression of such a multimarker panel, as well as the use of a PBC-expression based test to improve the diagnosis of ovarian cancer. The invention further relates to a method of determining the expression of at least one of the RPL21, RPL9 and / or SH3BGRL3 genes in a PBC sample of a subject as internal control.
Owner:ONCOLAB DIAGNOSTICS
Method for constructing tumor mutation load TMB panel and using method of tumor mutation load TMB panel
ActiveCN111951893ALower Sequencing CostsHigh speedMedical data miningMicrobiological testing/measurementCancer researchRelated gene
The invention discloses a method for constructing a tumor mutation load TMB panel. The method comprises the following steps: 1) acquiring transcriptome data of a tumor patient, and dividing the transcriptome data into a high TMB group and a low TMB group; 2) screening immune-related differential expression genes DEGs between the high TMB group and the low TMB group, and carrying out enrichment analysis; 3) carrying out immune cell infiltration analysis through Immune CellAI, and screening out potential key immune cells; 4) screening out tumor immunity related genes for prognosis of the patient; 5) establishing modules with different colors and a correlation matrix of immune traits possibly influencing prognosis; and 6) selecting genes with better correlation with macrophages, DC, MAIT andinfiltration integration in the WCGNA, and preliminarily obtaining a TMBIF gene panel. The invention also provides a using method of the tumor mutation load TMB panel. The tumor mutation load TMB panel constructed by the invention is low in sequencing cost and low in DNA input requirement in use, has shorter turnover time, can perform deeper sequencing, and improves the mutation detection sensitivity.
Owner:THE THIRD AFFILIATED HOSPITAL OF SUN YAT SEN UNIV
Kit for detecting idiopathic azoospermia and gene panel
PendingCN109811052AImprove the detection rateImprove work efficiencyMicrobiological testing/measurementDNA/RNA fragmentationMutation frequencyDetection rate
The invention relates to the field of gene detection technology, in particular to a kit for detecting idiopathic azoospermia and a gene panel. A small amount of (3 ml) peripheral blood from a patientis utilized to extract DNA, gene mutation of the patient is detected through library-establishing, high-throughput sequencing, etc., and through bioinformatics analysis, such as mutation frequency, mutation property, biological harm and conservative property, evaluation is performed to find 30 new morbigenous genetic mutations in the patient. Only 3 ml of peripheral blood of the patient is neededfor detection and 30 detection sites are covered, thereby improving the detection rate of the mutation, and 30 sites are carried out in one reaction system, to greatly reduce the workload, improve work efficiency, and save work time.
Owner:XUKANG MEDICAL SCI & TECH (SUZHOU) CO LTD
Gene Panel and probe for detecting generic cancer species and application of gene Panel and probe
InactiveCN114480660AEarly treatmentEarly detectionMicrobiological testing/measurementFermentationTumor targetTherapeutic effect
The invention discloses a gene Panel for detecting a generic cancer species, a probe and application of the gene Panel. The gene Panel comprises a tumor pathway related gene, a tumor genetic susceptibility gene, a tumor high-frequency mutant gene, a tumor targeted drug related gene, a tumor driving gene, an immune therapeutic effect related gene, a key DDR pathway related gene and other genes playing an important role in cancer generation and development. The gene Panel can be used for detecting all biomarkers corresponding to solid tumor targeted drugs approved by the State Drug Administration (NMPA) / American Food and Drug Administration (FDA) to appear in the market at a time, so that accurate treatment of tumors is fully guided. Prognosis of a subject can be evaluated, the recurrence risk of the subject is layered, and postoperative adjuvant therapy is guided; besides, hereditary tumor evaluation can be carried out on a subject, family genetic risks are prompted, and early discovery, early diagnosis and early treatment are achieved.
Owner:普瑞基准科技(北京)有限公司 +2
Methods and compositions for diagnosis of glioblastoma or a subtype thereof
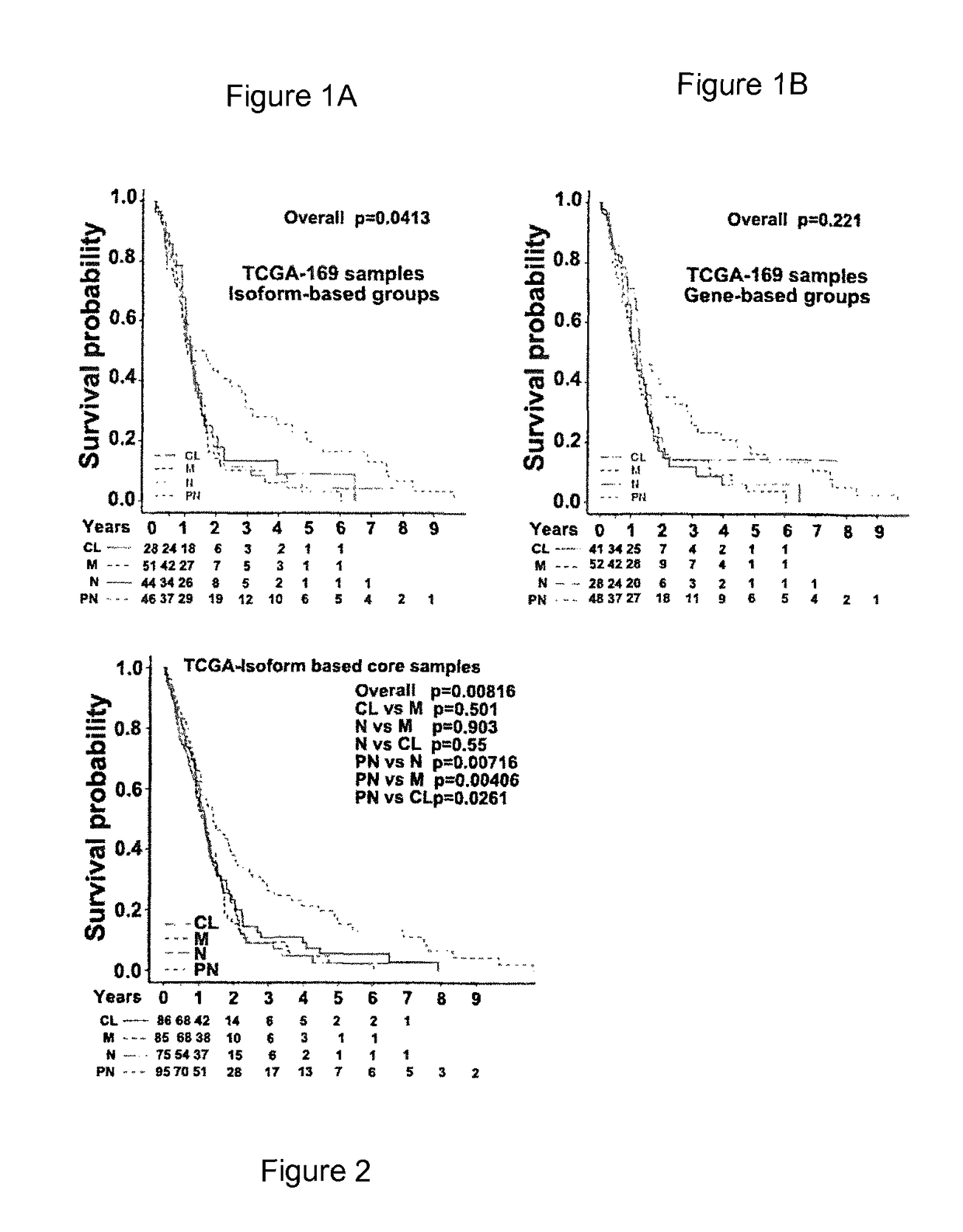
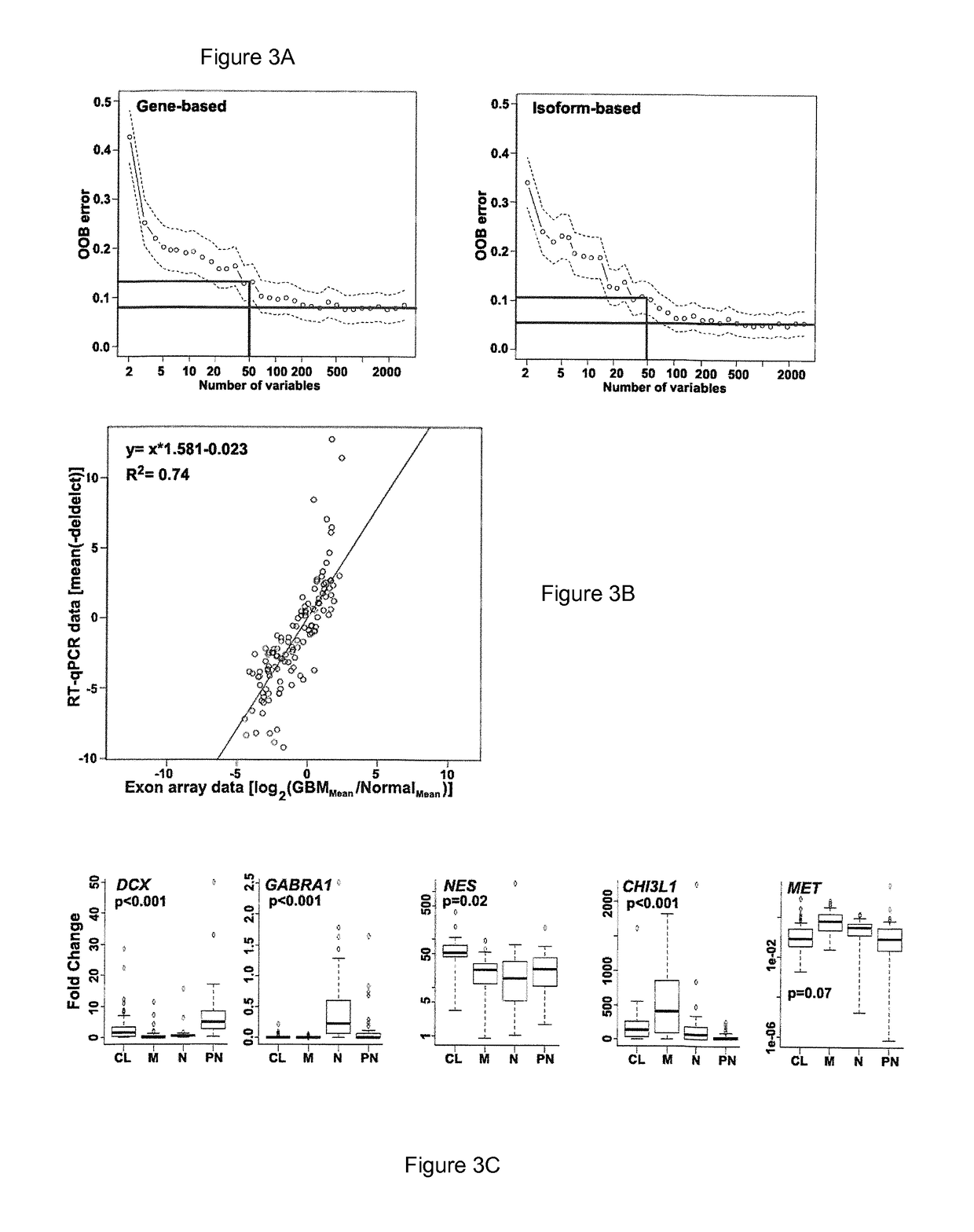
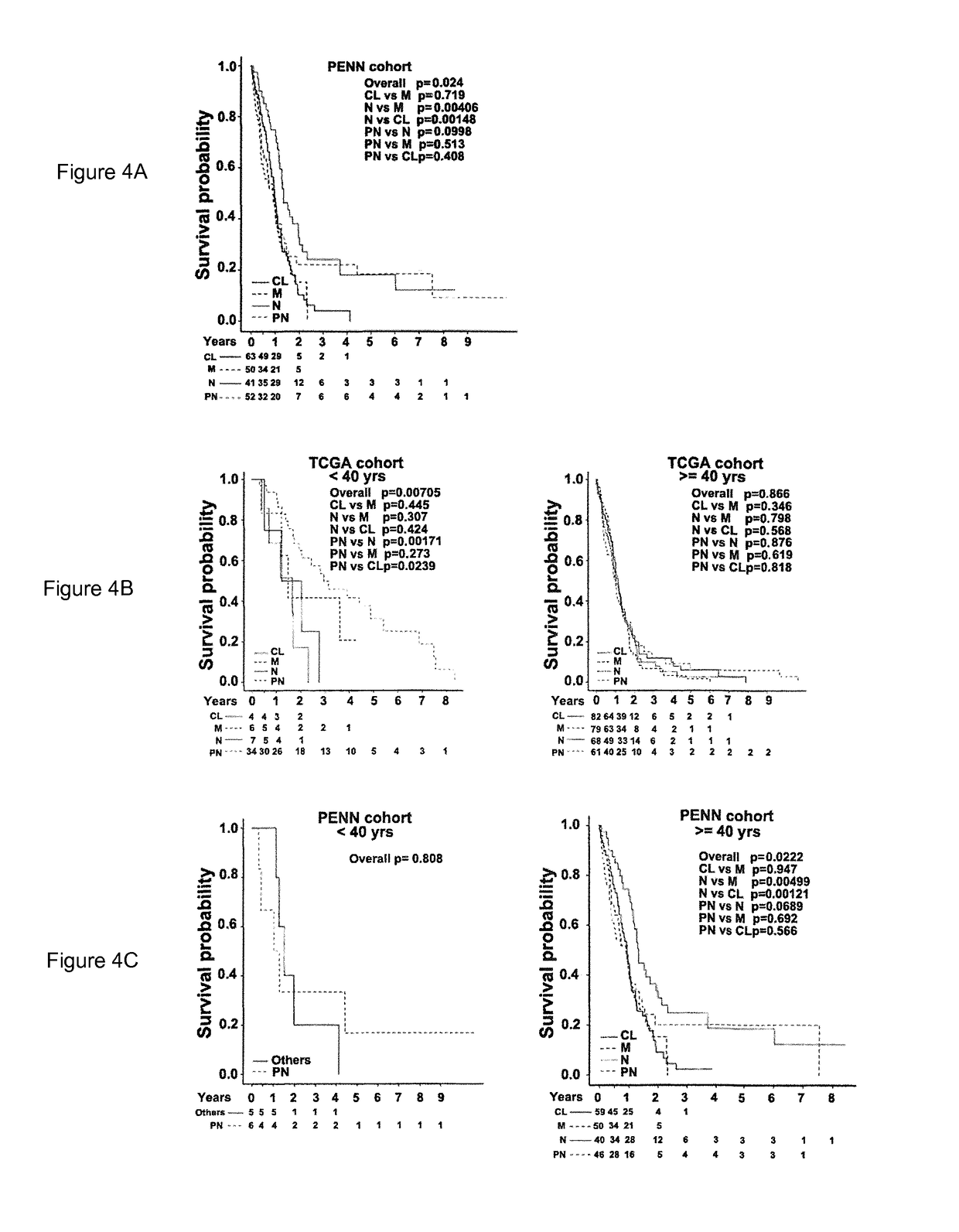
An isoform-level gene panel is disclosed that can accurately classify a glioblastoma subtype from a tumor sample. Such an isoform level gene panel comprises the 121 to 214 target isoforms identified in Table 1. Also disclosed are reagents for quantitatively detecting the expression or activity of the target isoforms of Table 1 in a patient sample. For example, such ligands can be PCR primer and probes sets. This isoform-level gene panel and reagents for detection of the isoforms are useful in an isoform-level assay for diagnosis of the molecular subtype of a glioblastoma in a patient. The assay employs algorithms and a novel computer program that performs the functions of FIG. 8. In one aspect, the assay is a high-throughput format.
Owner:THE WISTAR INST OF ANATOMY & BIOLOGY +1
Gene panel participating in liver regeneration
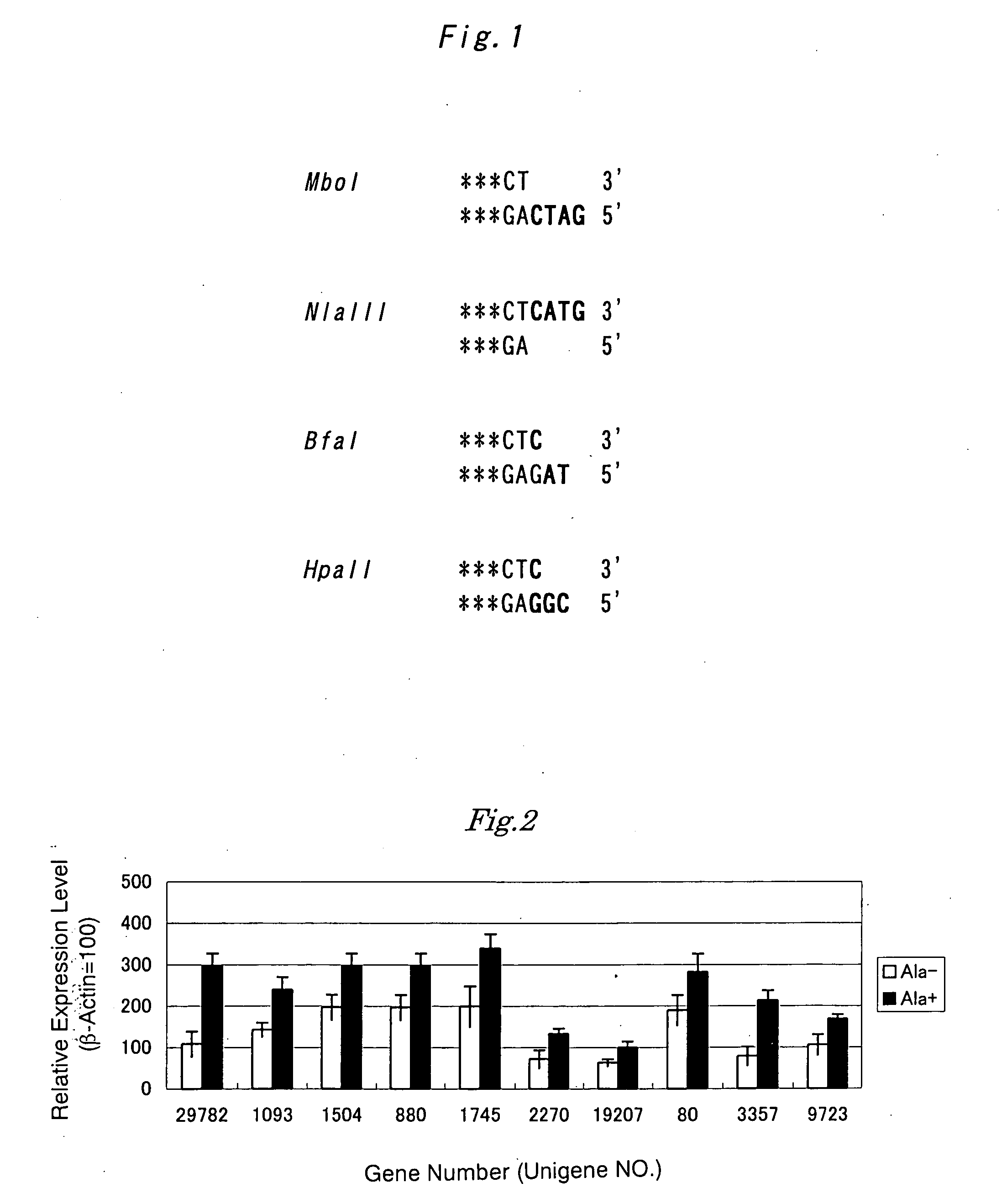
InactiveUS20060105329A1To promote metabolismImprove featuresMicrobiological testing/measurementDigestive systemGene expression levelCancer research
A gene panel comprising a group of genes of which expression levels change in hepatocytes during liver regeneration as compared with those in a normal state is produced by the following steps: (a) the step of measuring expression levels of various genes in hepatocytes of a model animal in a normal state and expression levels of the genes during liver regeneration; (b) the step of comparing the expression levels, respectively; and (c) the step of identifying a group of genes of which expression levels change during liver regeneration.
Owner:AJINOMOTO CO INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com