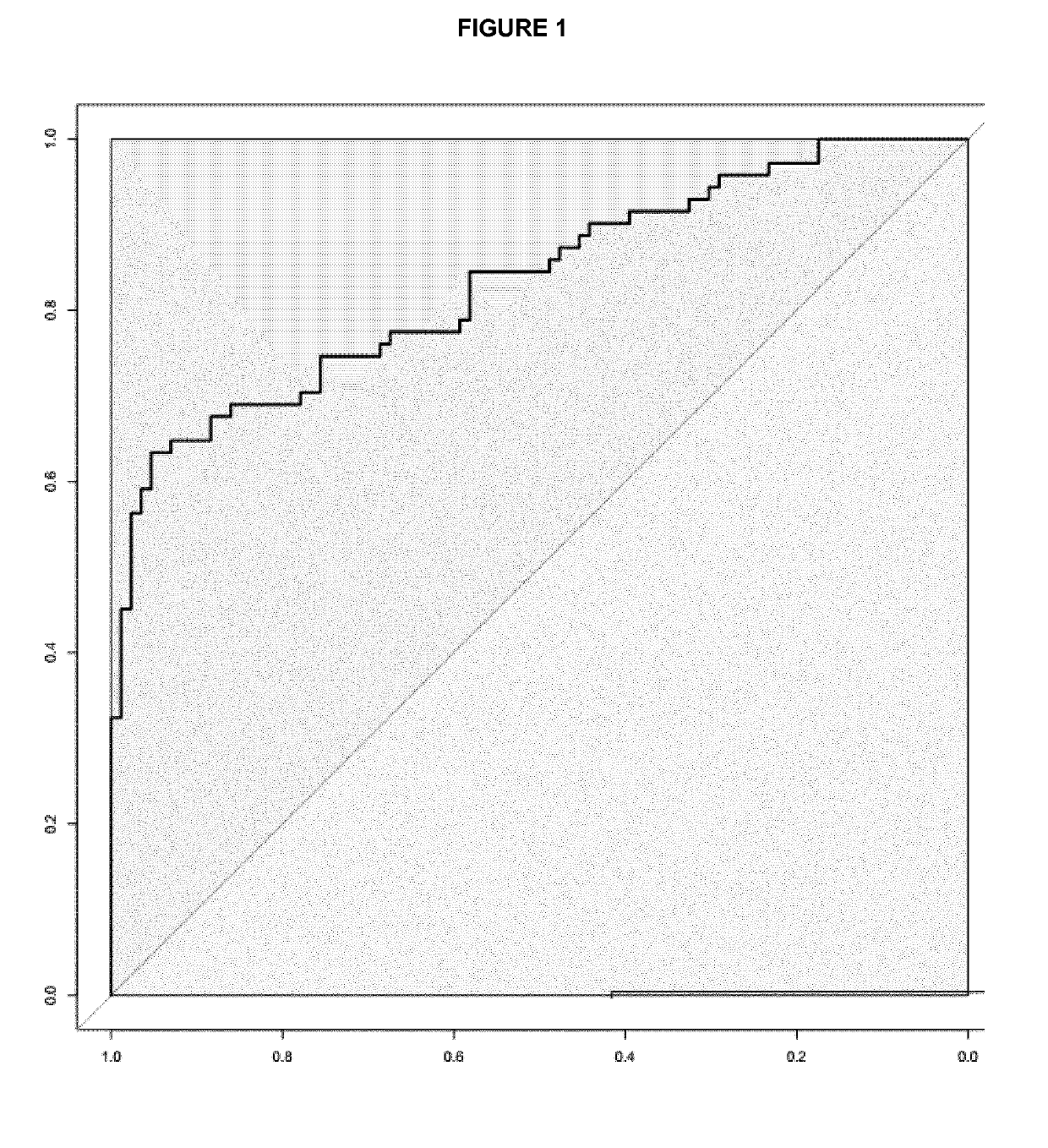
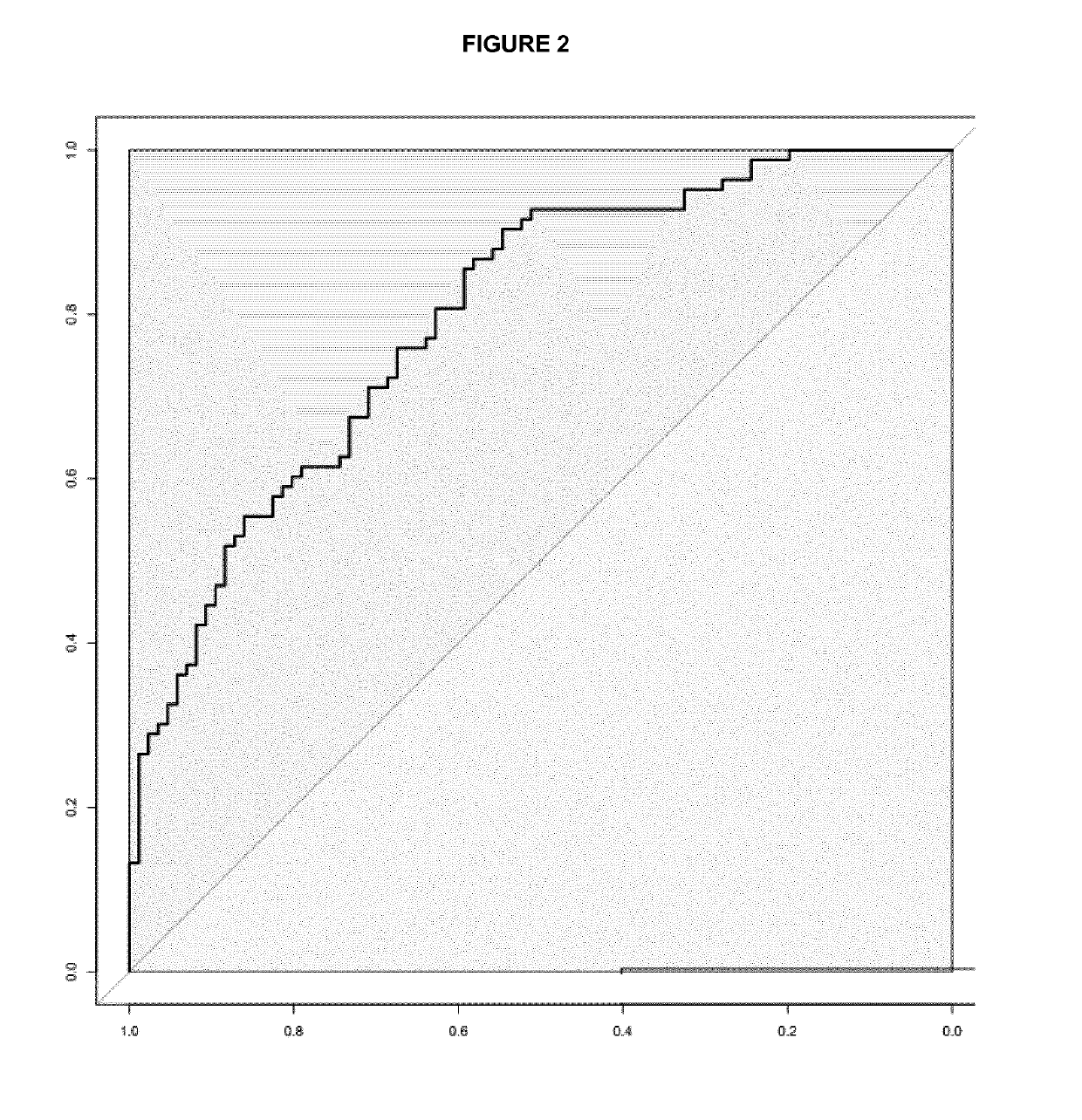
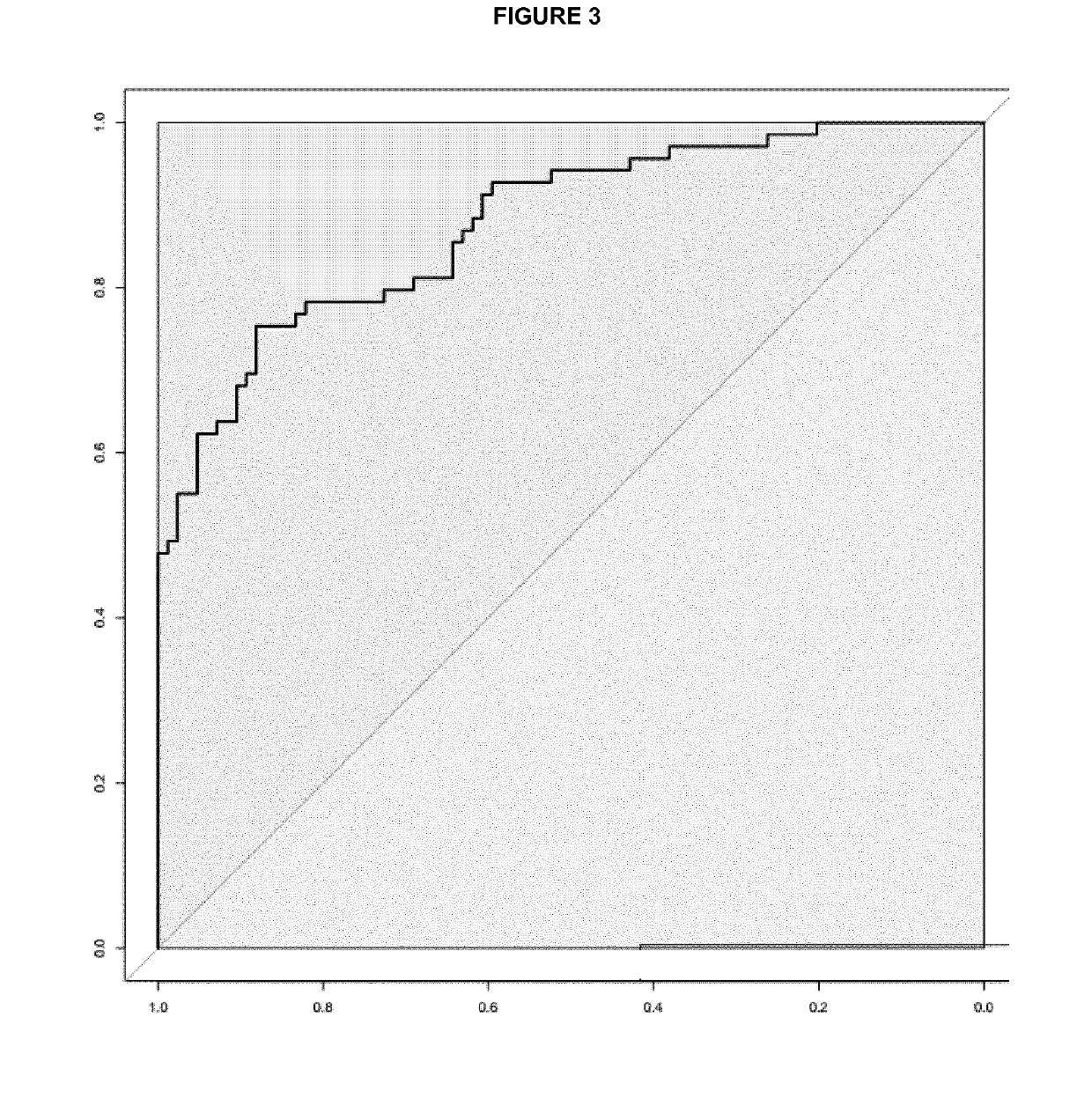
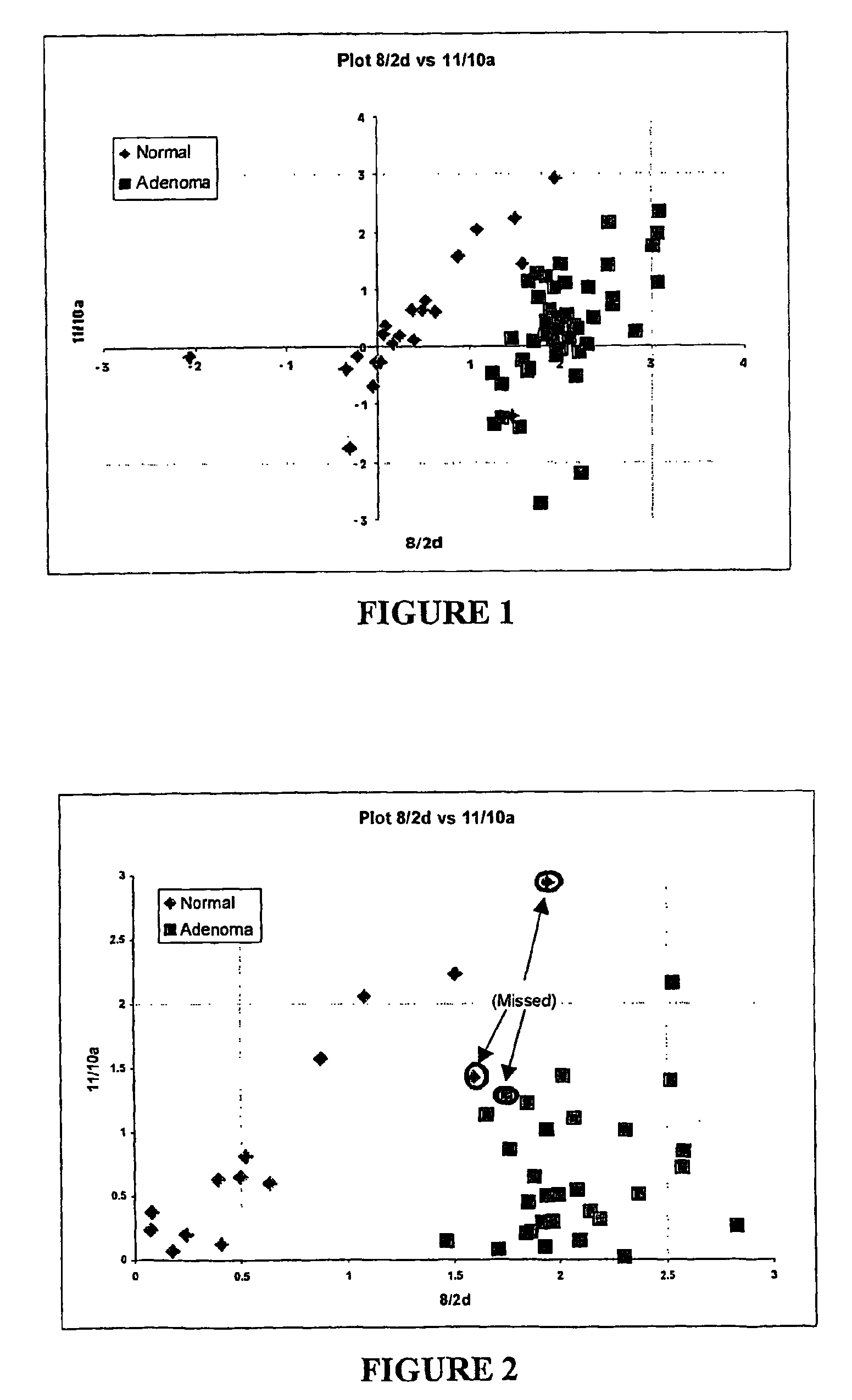
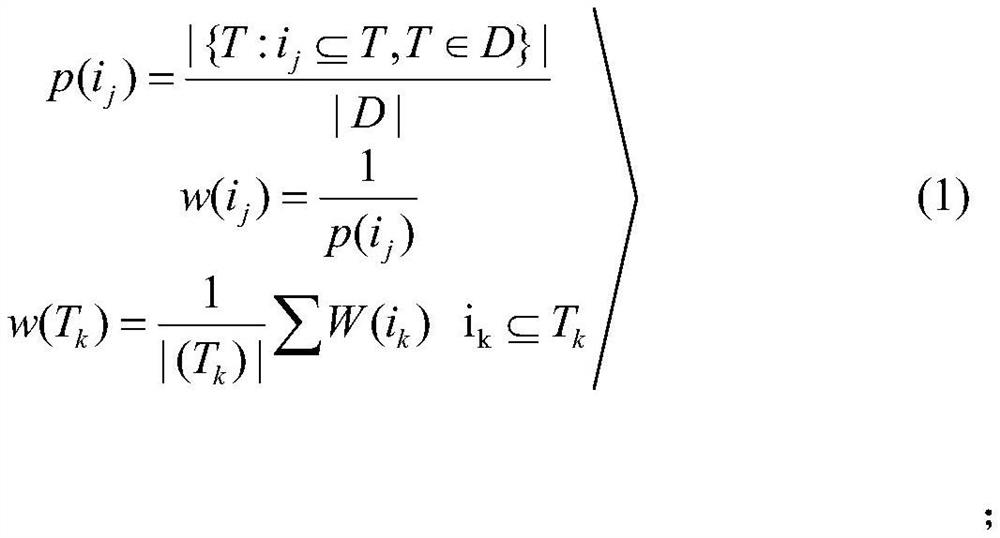Patents
Literature
68 results about "Colorectal adenoma" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Tubulovillous adenoma, TVA, is a type of polyp that grows in the colon and other places in the gastrointestinal tract and sometimes in other parts of the body. TVAs are considered to have a higher risk of becoming malignant (cancerous) than tubular adenomas.
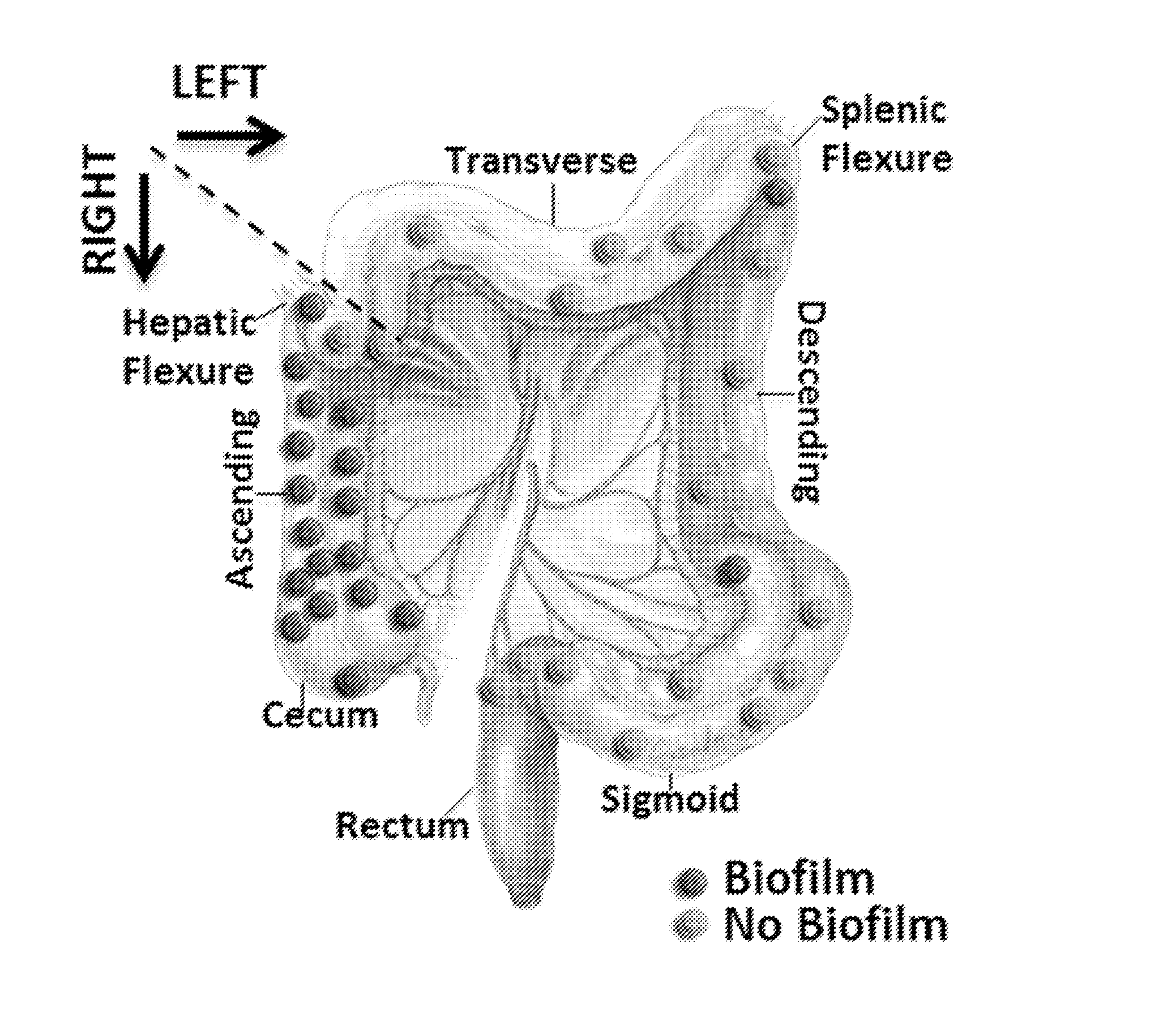
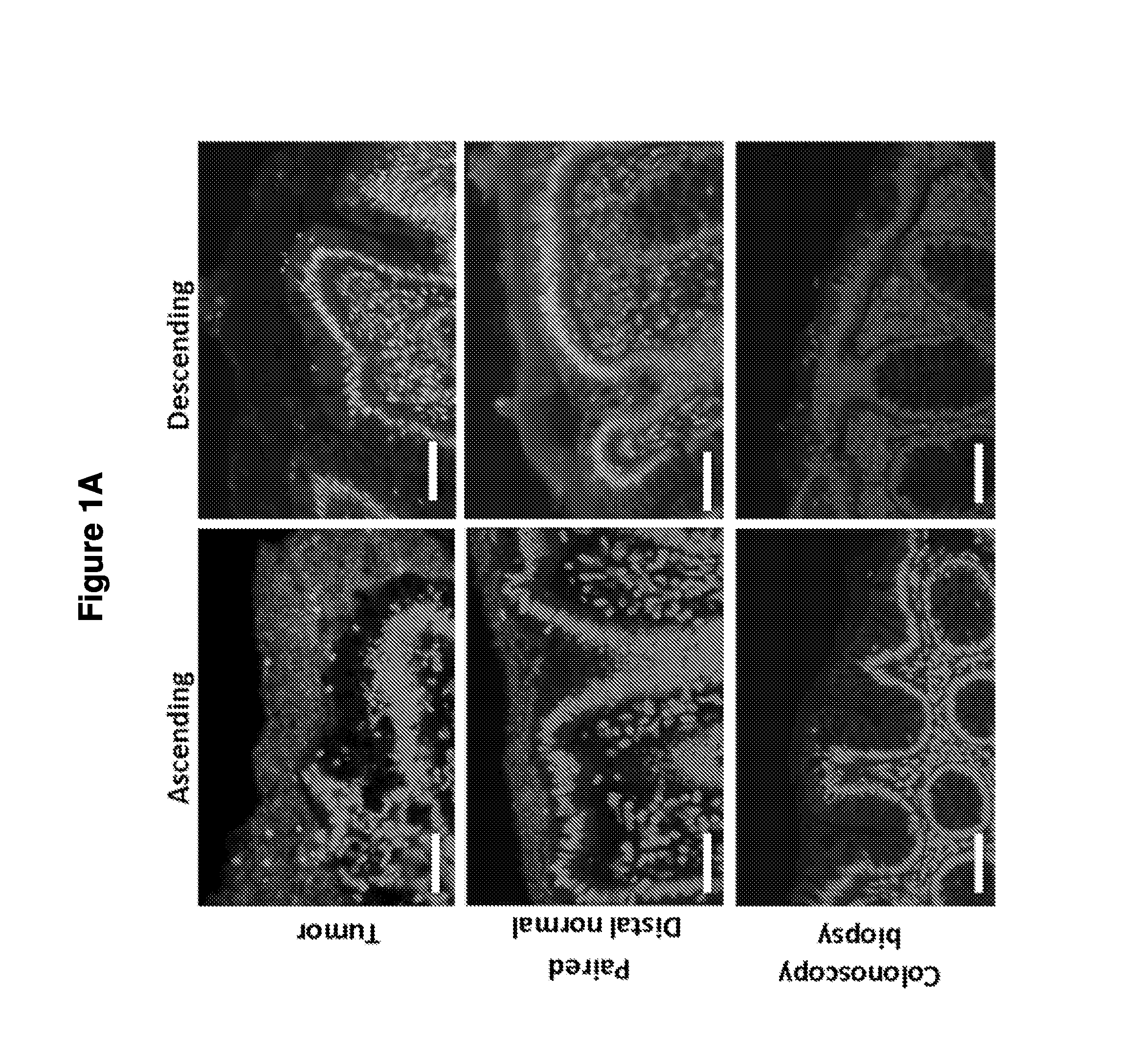
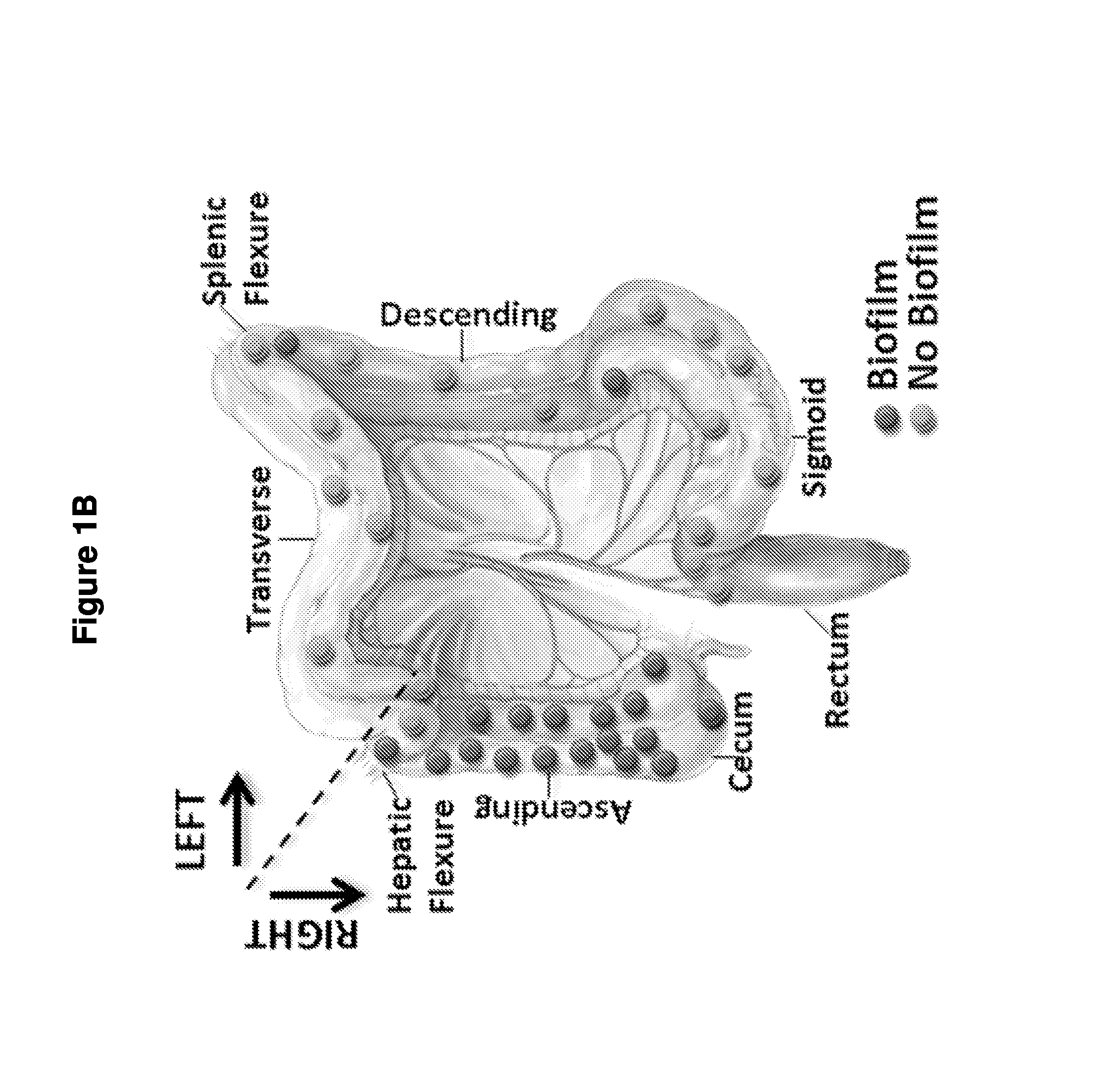
Biofilm formation to define risk for colon cancer
ActiveUS20160223553A1Preventing and diminishing developmentIncreased proliferationMicrobiological testing/measurementUnknown materialsFecesIncreased risk
Methods of identifying subjects at increased risk of cancer, based upon detection of biofilms and / or biofilm-associated microbes within a subject, are disclosed. Therapies designed to prevent formation and / or reduce the size of biofilms in a subject identified to be at increased risk of cancer based upon detection of biofilms and / or biofilm-associated microbes are disclosed. In particular embodiments, the invention provides for identification of a subject at elevated risk of developing or having colorectal cancer and / or a colorectal adenoma, based upon detection of a biofilm and / or biofilm-associated bacteria within the gastrointestinal tract of the subject (optionally, within a biopsy specimen and / or stool sample of such subject). Therapies involving administration of an antibiotic agent and / or a probiotic agent to a subject, to prevent or reduce biofilm formation within the gastrointestinal tract of the subject, optionally provided in combination with additional cancer therapy, are also disclosed.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Method for detection of colorectal tumor
InactiveUS20120034605A1High sensitivitySimply detectMicrobiological testing/measurementTwist HomologTWIST1 gene
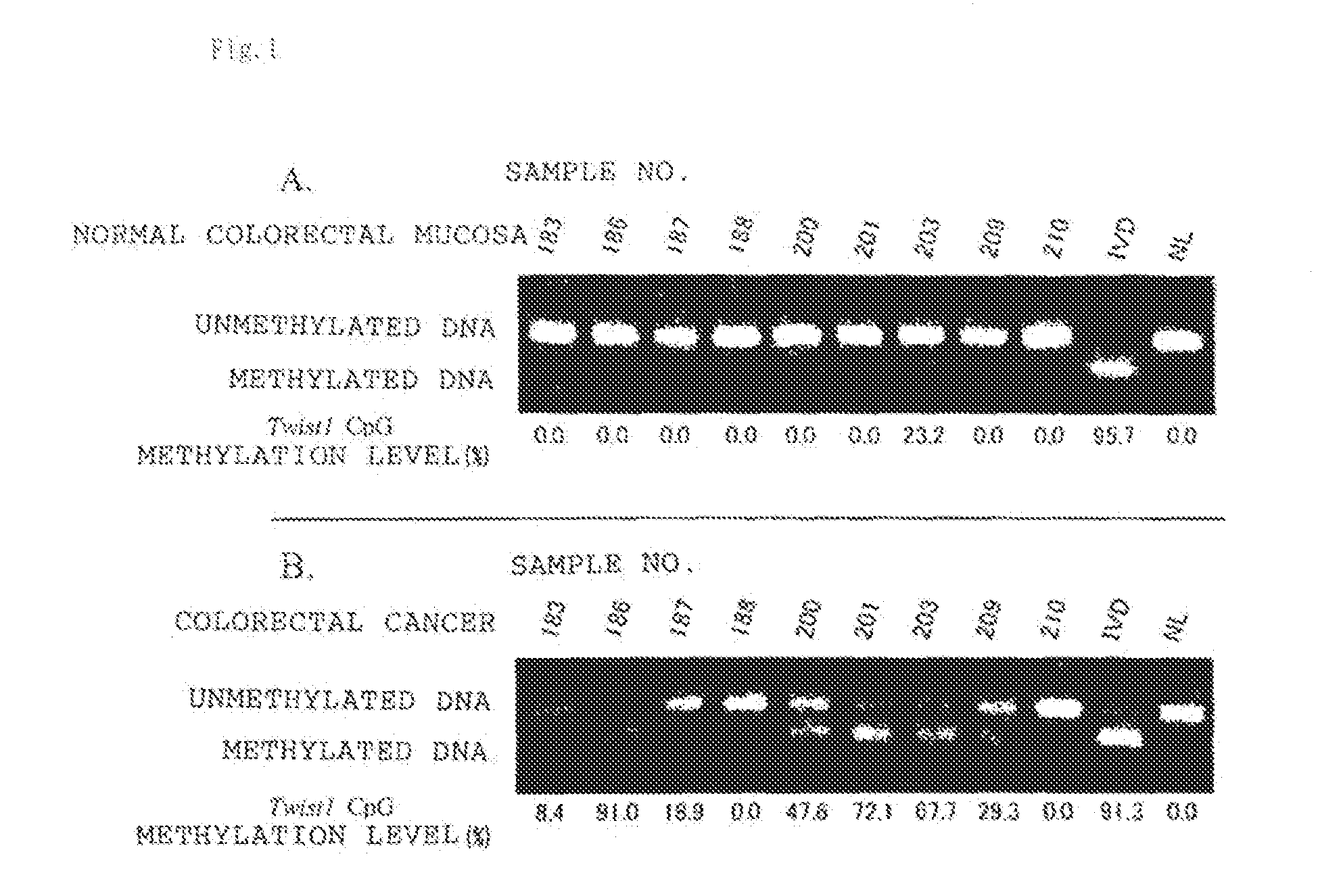
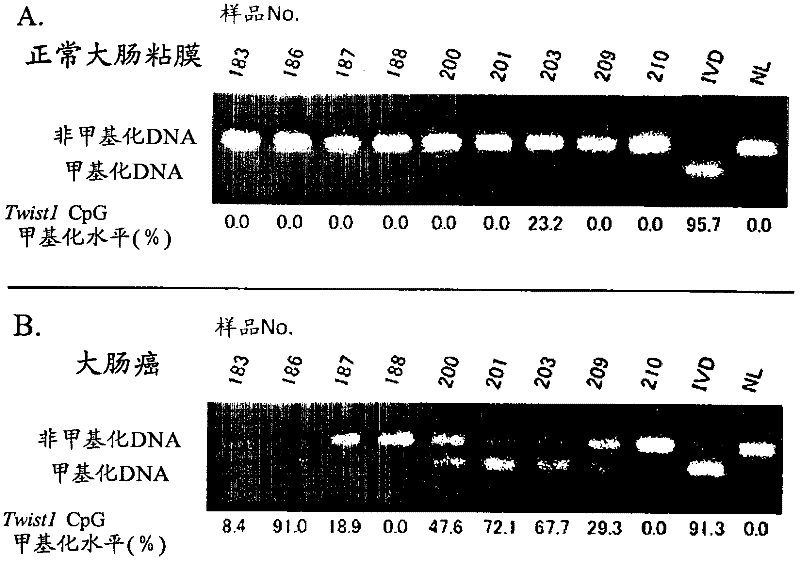
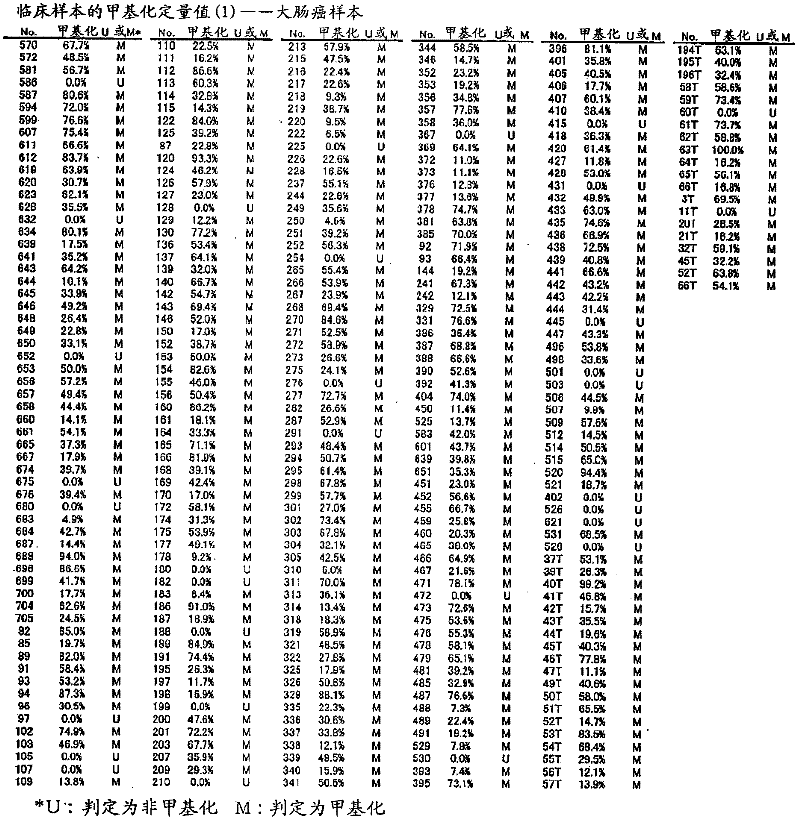
Disclosed is a method for determining the presence or absence of a colorectal tumor, specifically colorectal cancer or colorectal adenoma, with high sensitivity and high specificity by employing the methylation of DNA as a measure. Also disclosed is a kit for carrying out the method. Specifically, measurement is made on the degree of methylation of one or more CpG sequences contained in the region lying between positions -477 to -747, more preferably a CGCG sequence contained in the region lying between positions -688 to -691, in TWIST1 gene (Homo sapiens twist homolog 1; Drosophila gene) located on the genome sequence of a test cell.
Owner:A T LT
Bile preparations for colorectal disorders
InactiveUS20070072828A1Reduce recurrenceProlong lifeBiocideOrganic active ingredientsColorectal adenomaWater soluble
The present disclosure relates to methods and compositions to ameliorate or treat at least one symptom of colorectal cancer and / or adenomatous polyposis coli (APC). For example, some embodiments of the methods and compositions may reduce recurrence of colorectal adenomas and / or extend the life of a subject having colorectal cancer and / or APC. Some embodiments of the disclosure include maintaining a the total body weight in a subject having colorectal cancer and / or APC. According to some embodiments, a method of the disclosure may include administering a bile acid composition to a subject. A bile acid composition may include, in some embodiments, an aqueous solution that is free or substantially free of precipitates or particles. A aqueous solution may include (1) a bile acid, an aqueous soluble derivative of a bile acid, a bile acid salt, and / or 7-ketolithocholic acid, (2) a carbohydrate, and (3) water. An aqueous composition may further include an alkali.
Owner:YOO SEO HONG
Methods for the detection of colorectal tumors
InactiveCN102292458AEasy to detectHigh sensitivityMicrobiological testing/measurementRecombinant DNA-technologyDNA methylationTwist Homolog
Disclosed is a method for determining the presence or absence of a colorectal tumor, specifically colorectal cancer or colorectal adenoma, with high sensitivity and high specificity by employing the methylation of DNA as a measure. Also disclosed is a kit for carrying out the method. Specifically, measurement is made on the degree of methylation of one or more CpG sequences contained in the region lying between positions -477 to -747, more preferably a CGCG sequence contained in the region lying between positions -688 to -691, in TWIST1 gene (Homo sapiens twist homolog 1; Drosophila gene) located on the genome sequence of a test cell.
Owner:A T LT
Methods and tools for discriminating colorectal adenomas and adenocarcinomas
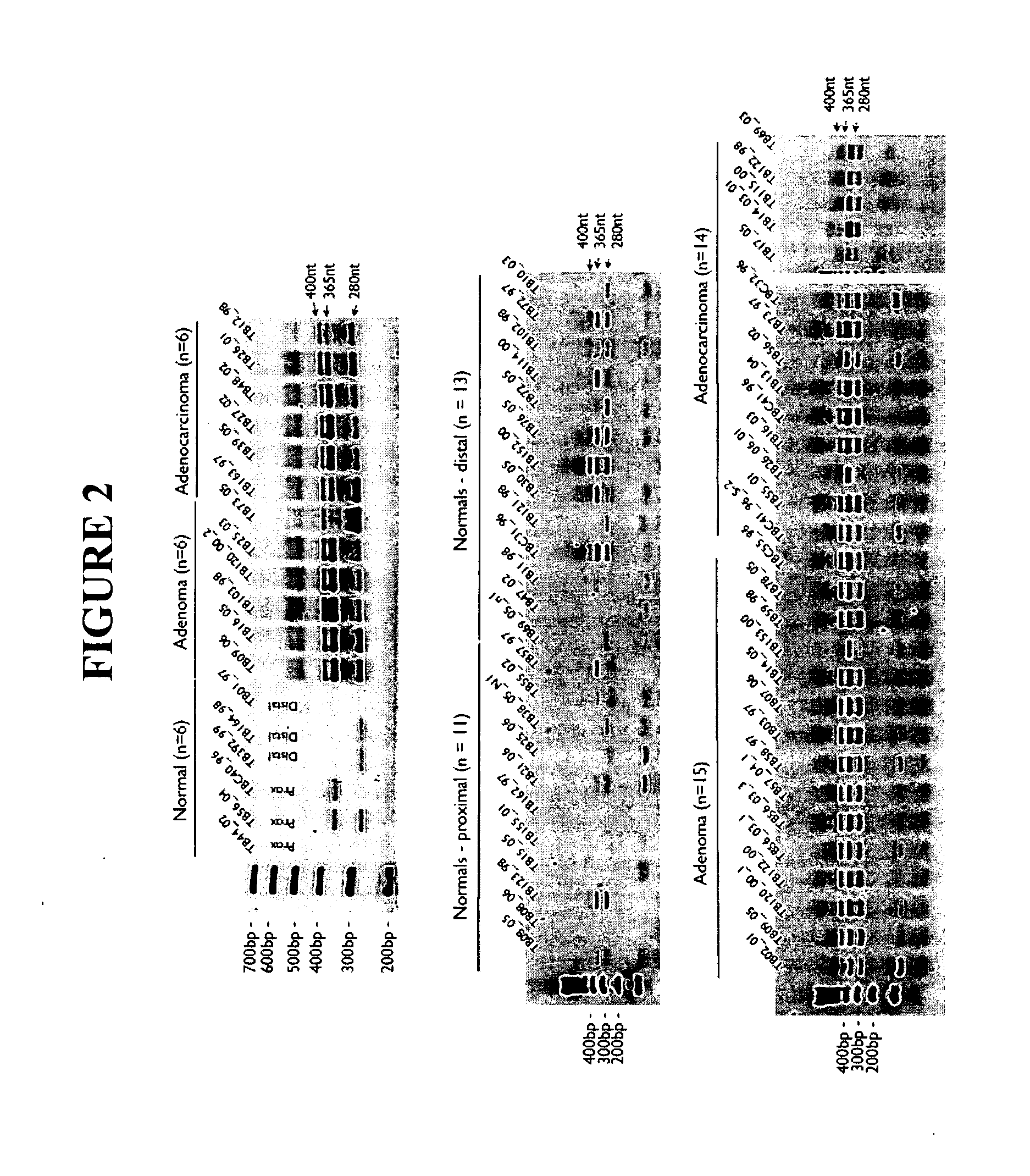
InactiveUS20100304374A1Altered level of expressionImprove expression levelMicrobiological testing/measurementMaterial analysisIn vivoAdenoma
The present invention relates to the differential expression of genes in colorectal adenoma and adenocarcinoma cells and their correlation with chromosomal aberrations. The present invention provides tools for detecting chromosomal aberrations linked to progression of adenomas into adenocarcinoma cells. The present invention discloses methods and tools for in vivo and in vitro diagnosis of colorectal tumours.
Owner:VER VOOR CHRISTELIJK HOGER ONDERWIJS WETENSCHAPPELIJK ONDERZOEK & PATIENTENZORG
Use of set of biomarkers in preparation of colorectal cancer diagnostic reagents
InactiveCN106468714AHigh sensitivityImprove featuresOmicsBiological testingDiagnosis earlyTest sample
The invention discloses the use of a set of biomarkers in the preparation of colorectal cancer diagnostic reagents, and further proposes a method of using biomarkers for distinguishing between normal intestinal cells and colorectal cancer cells. The method comprises: a) separating a test sample from a biopsy specimen of a subject; b) separating biological samples containing normal colorectal tissue cells or tissues from the biopsy specimens or the subject or family members thereof; c) analyzing the content of biomarker proteins of the above samples in a) and b); and d) comparing the content of biomarker proteins in normal and colorectal cancer cells or tissues. The invention also discloses a kit for detecting the level of biomarkers in a specimen. The method of the invention can be combined with other diagnostic methods to detect colorectal adenoma / colorectal cancer and improve the overall sensitivity and specificity, and can be also applied to early diagnosis and monitoring.
Owner:POOCHON SCI LLC
Kit for distinguishing colorectal adenomas from colorectal cancer
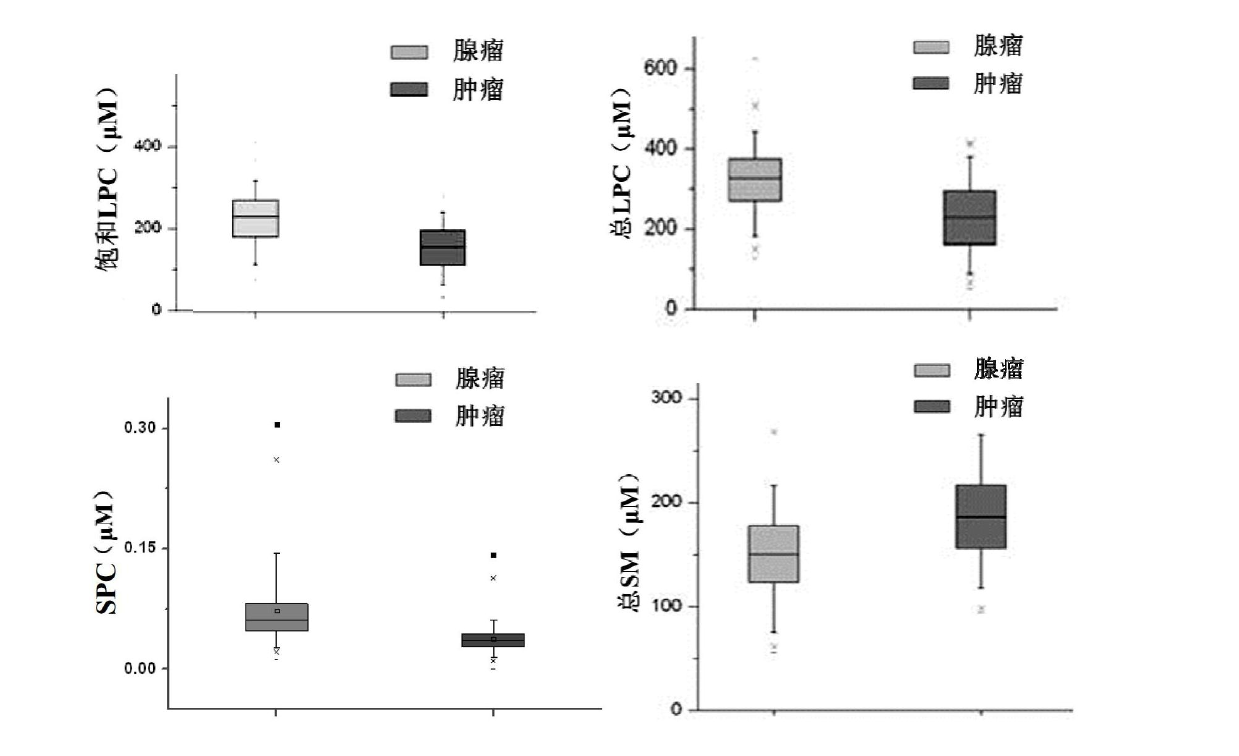
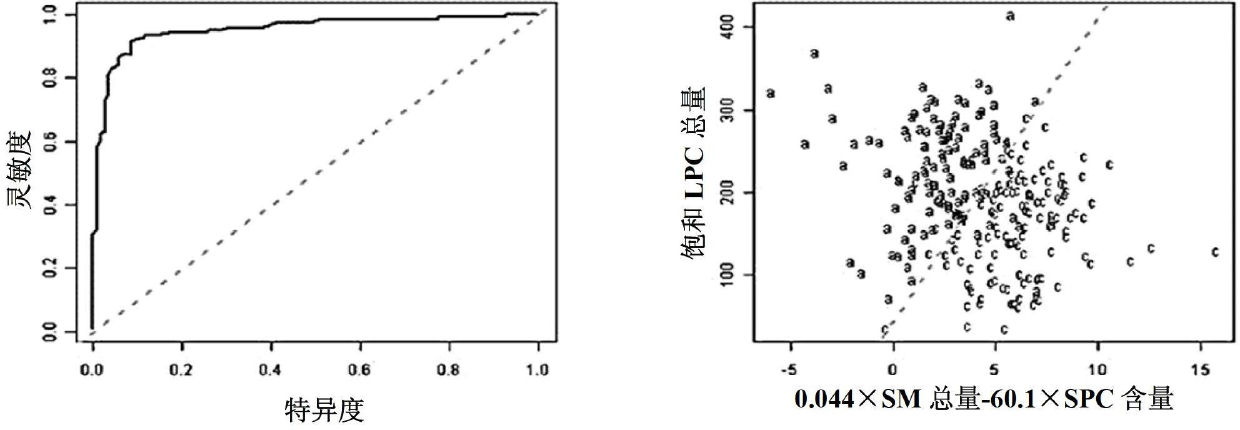
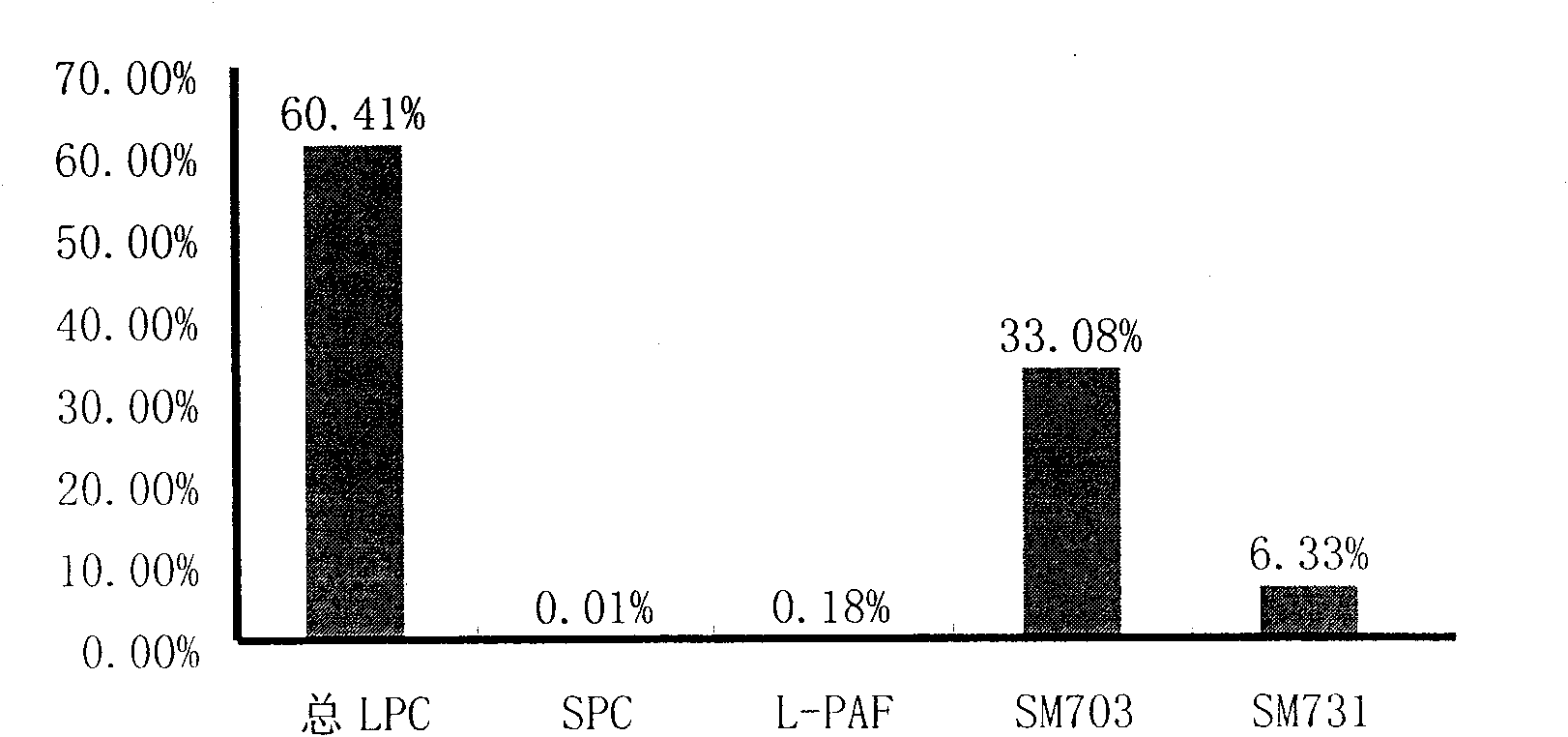
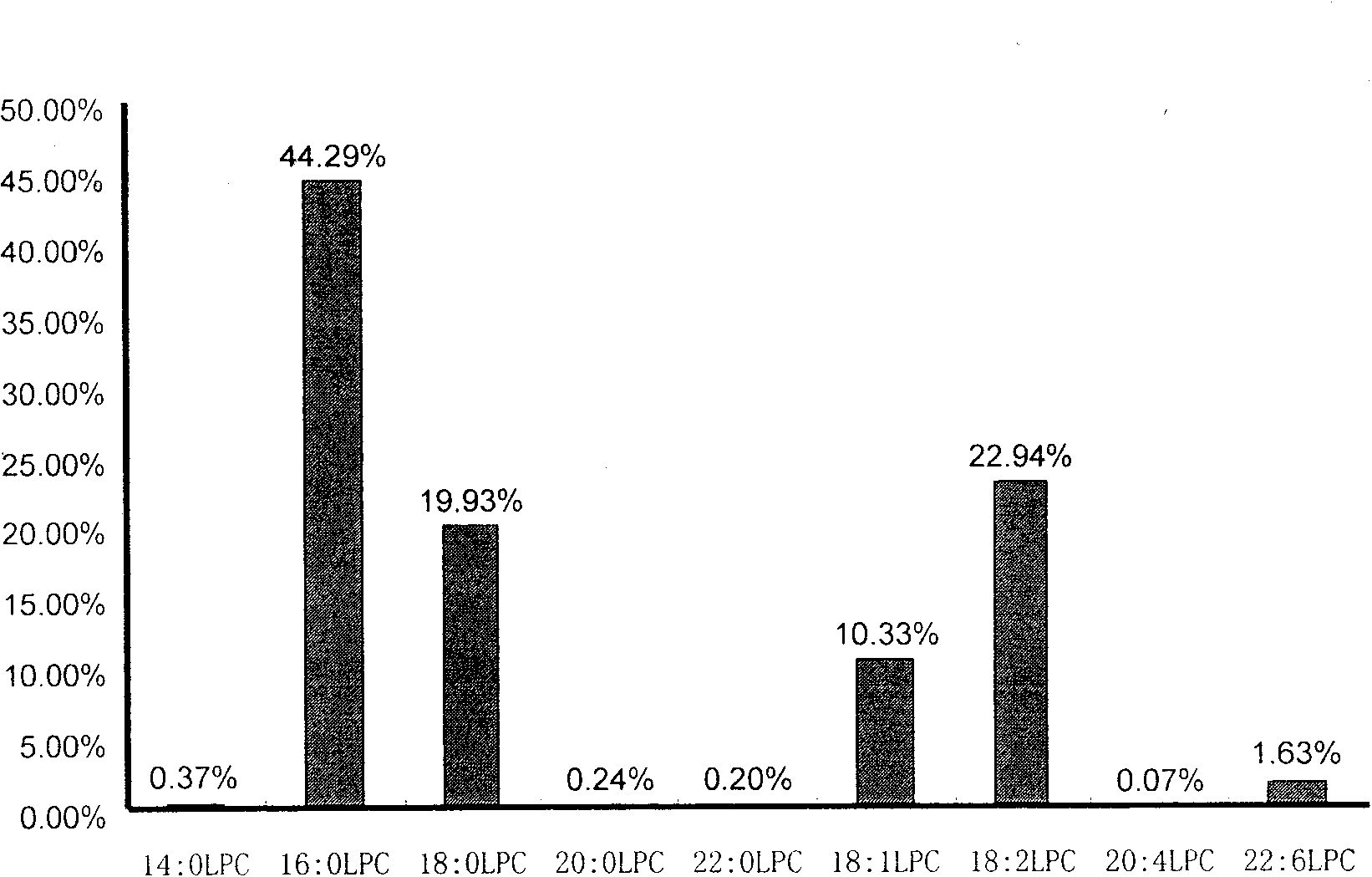
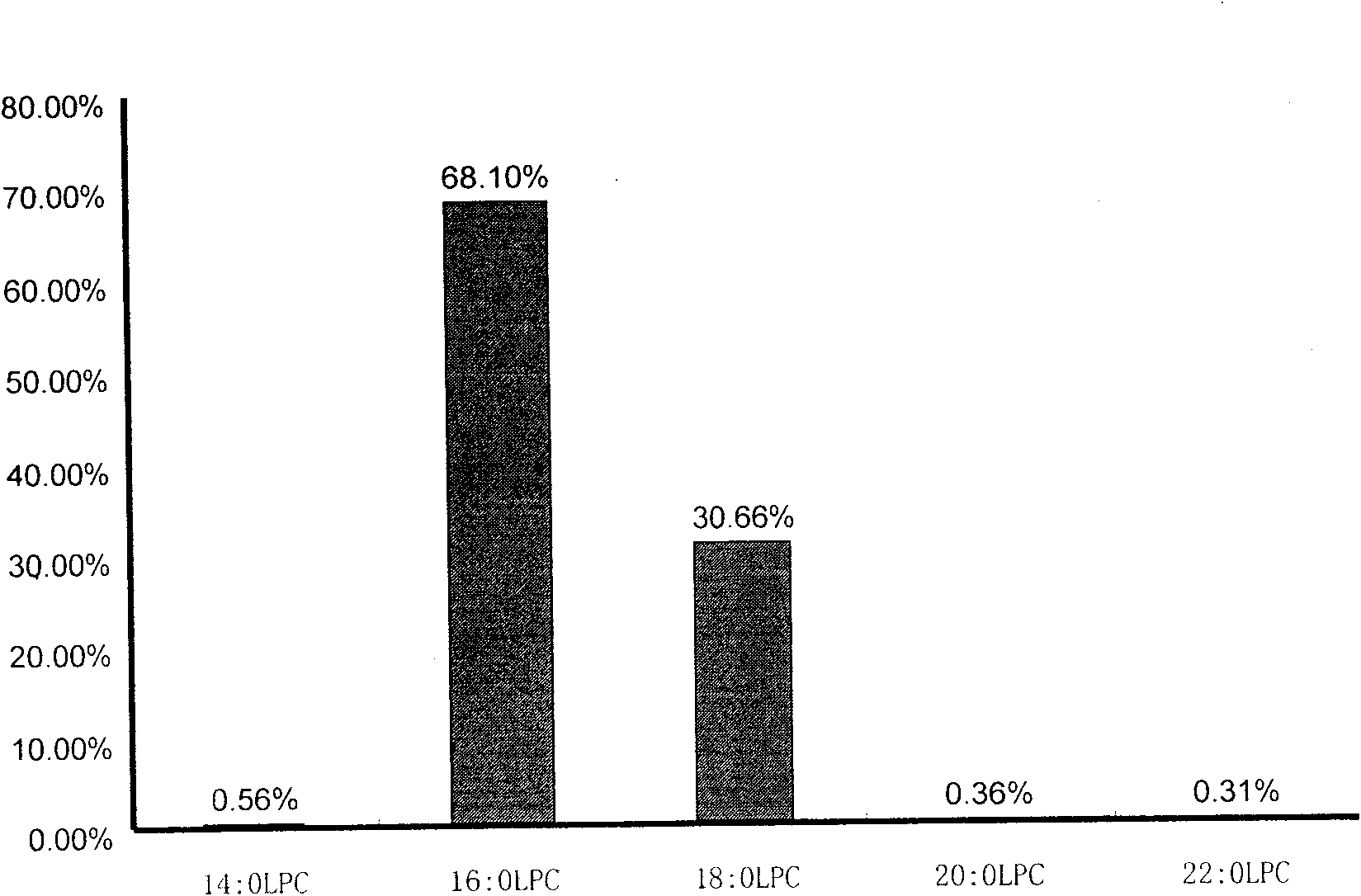
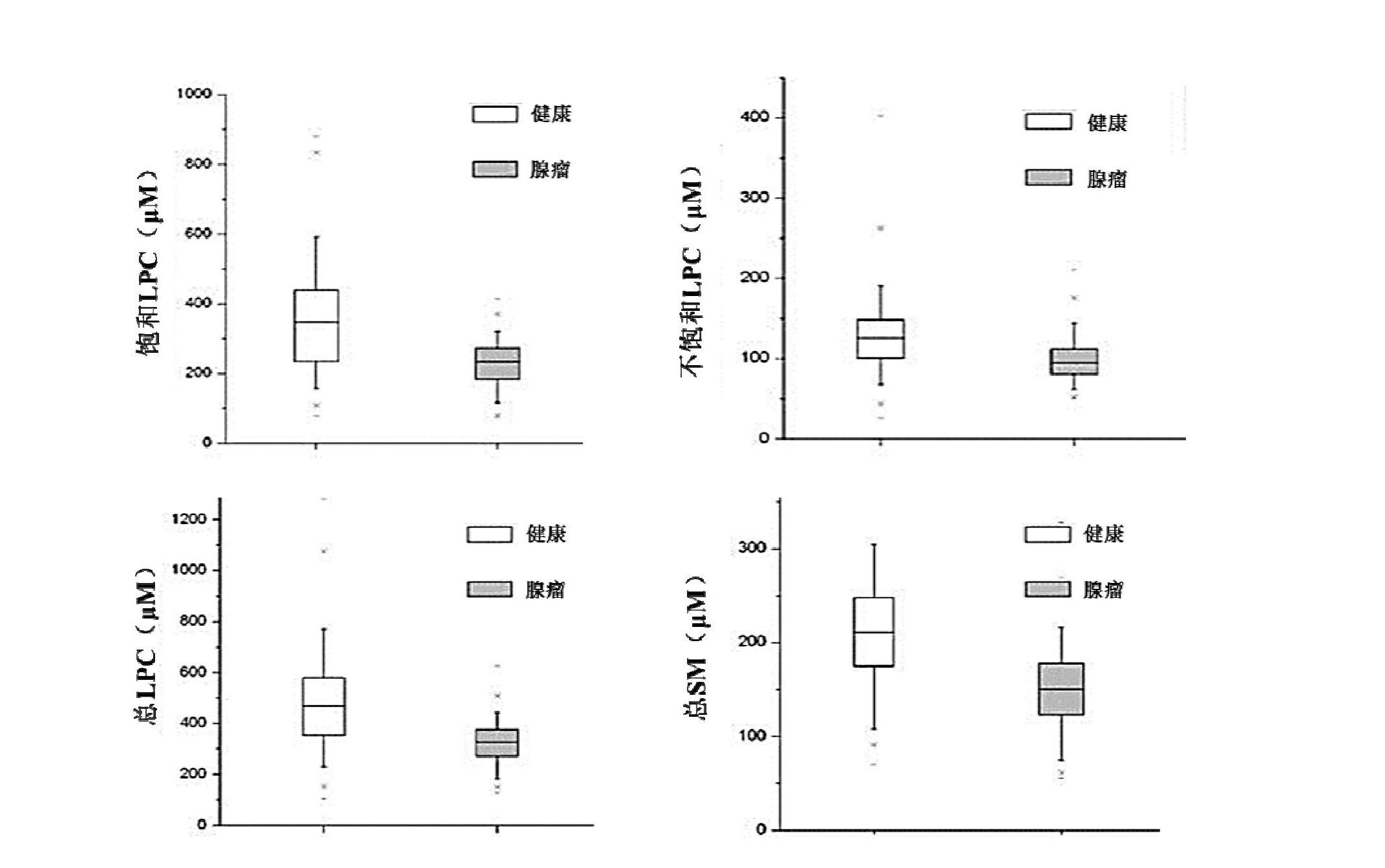
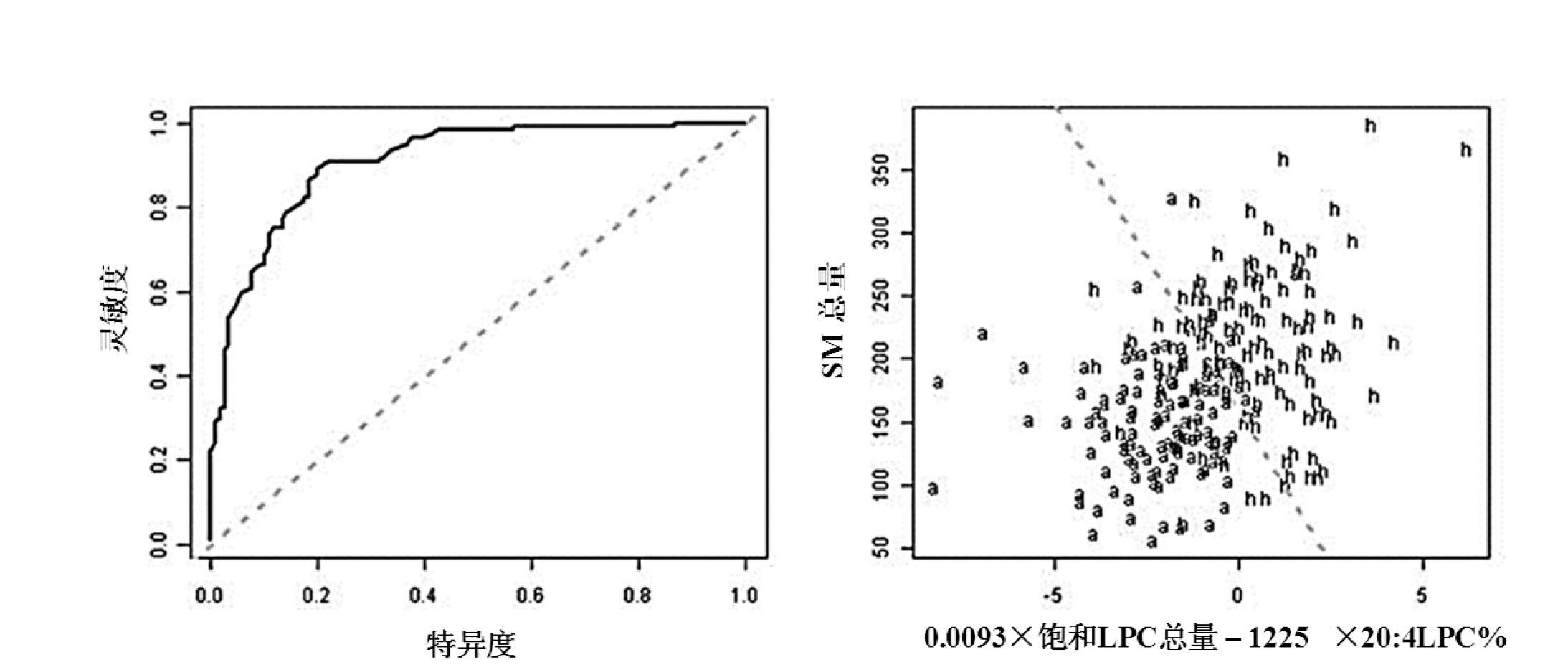
The invention discloses a kit for distinguishing colorectal adenomas from colorectal cancer. The kit for distinguishing or assisting in distinguishing the colorectal adenomas from the colorectal cancer comprises a device for detecting phospholipids content and a contrast card established by the phospholipids content, wherein phospholipids are sphingosylphosphorylcholine (SPC), 16:0 sphingomyelin (SM), 18:0SM, 14:0 lysophosphatidylcholine (LPC), 16:0LPC, 18:0LPC, 20:0LPC and 22:0LPC. Saturated LPC, SPC and SM are taken as markers, and a molecular model for distinguishing the colorectal adenomas from the colorectal cancer is established, and has colorectal cancer distinguishing sensitivity of 90 percent and colorectal cancer distinguishing specificity of 93 percent.
Owner:BEIJING NORMAL UNIVERSITY
Bile preparations for colorectal disorders
The present disclosure relates to methods and compositions to ameliorate or treat at least one symptom of colorectal cancer and / or adenomatous polyposis coli (APC). For example, some embodiments of the methods and compositions may reduce recurrence of colorectal adenomas and / or extend the life of a subject having colorectal cancer and / or APC. Some embodiments of the disclosure include maintaining a the total body weight in a subject having colorectal cancer and / or APC. According to some embodiments, a method of the disclosure may include administering a bile acid composition to a subject. A bile acid composition may include, in some embodiments, an aqueous solution that is free or substantially free of precipitates or particles. A aqueous solution may include (1) a bile acid, an aqueous soluble derivative of a bile acid, a bile acid salt, and / or 7-ketolithocholic acid, (2) a carbohydrate, and (3) water. An aqueous composition may further include an alkali.
Owner:柳署弘
Leveraging sequence-based fecal microbial community survey data to identify composite biomarker for colorectal cancer
PendingCN110637097AMicrobiological testing/measurementSequence analysisFecesIntestinal microorganisms
The present disclosure provides fecal microbial markers for diagnosing colorectal cancer and colorectal adenoma. The present disclosure also provides methods for diagnosing colorectal cancer and colorectal adenoma using these intestinal microbial markers.
Owner:SECOND GENOME +1
Method of examining colon cancer and colon adenoma
InactiveUS20070196874A1Biological material analysisImmunoglobulins against cell receptors/antigens/surface-determinantsNon cancerIsozyme
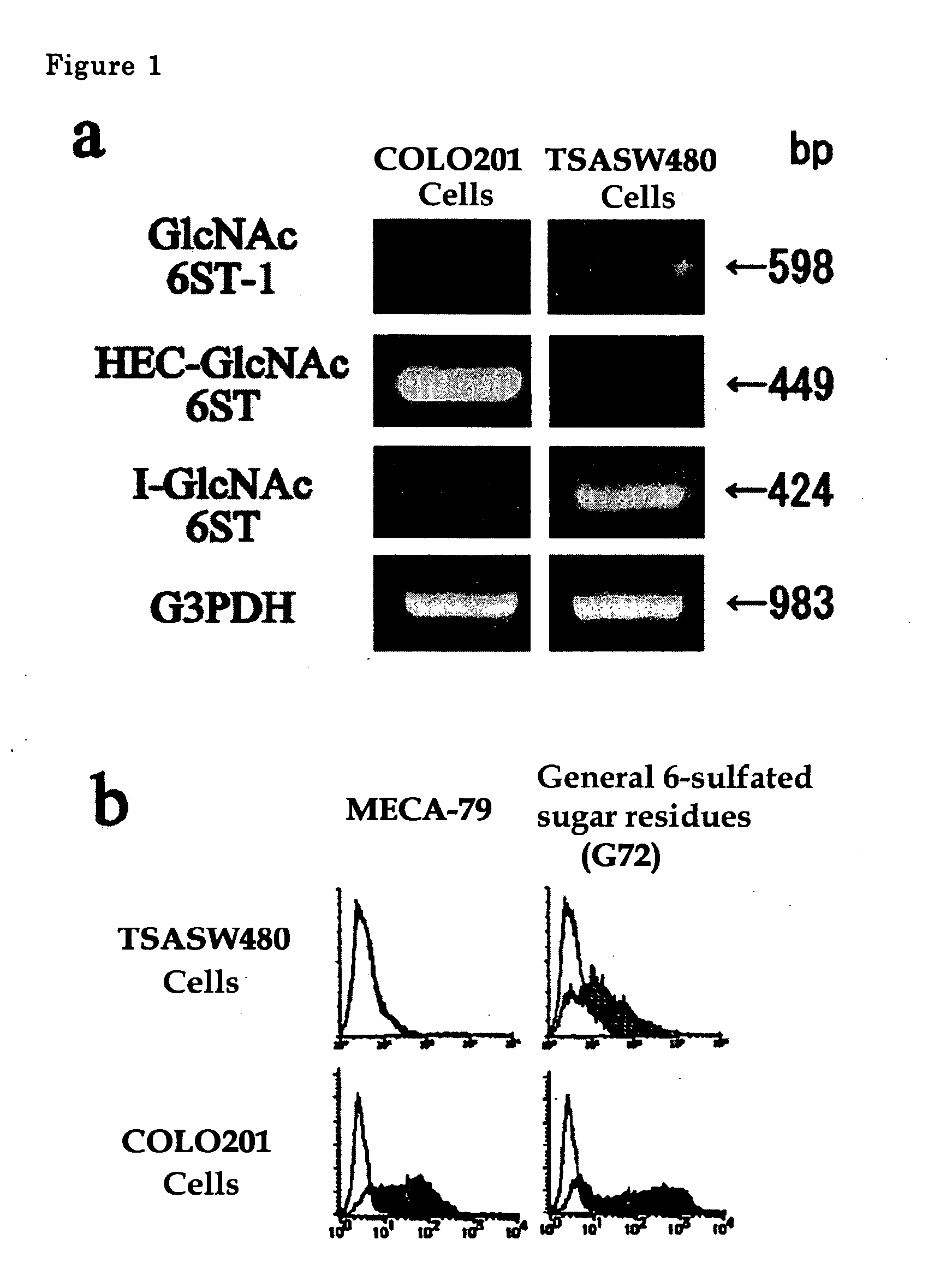
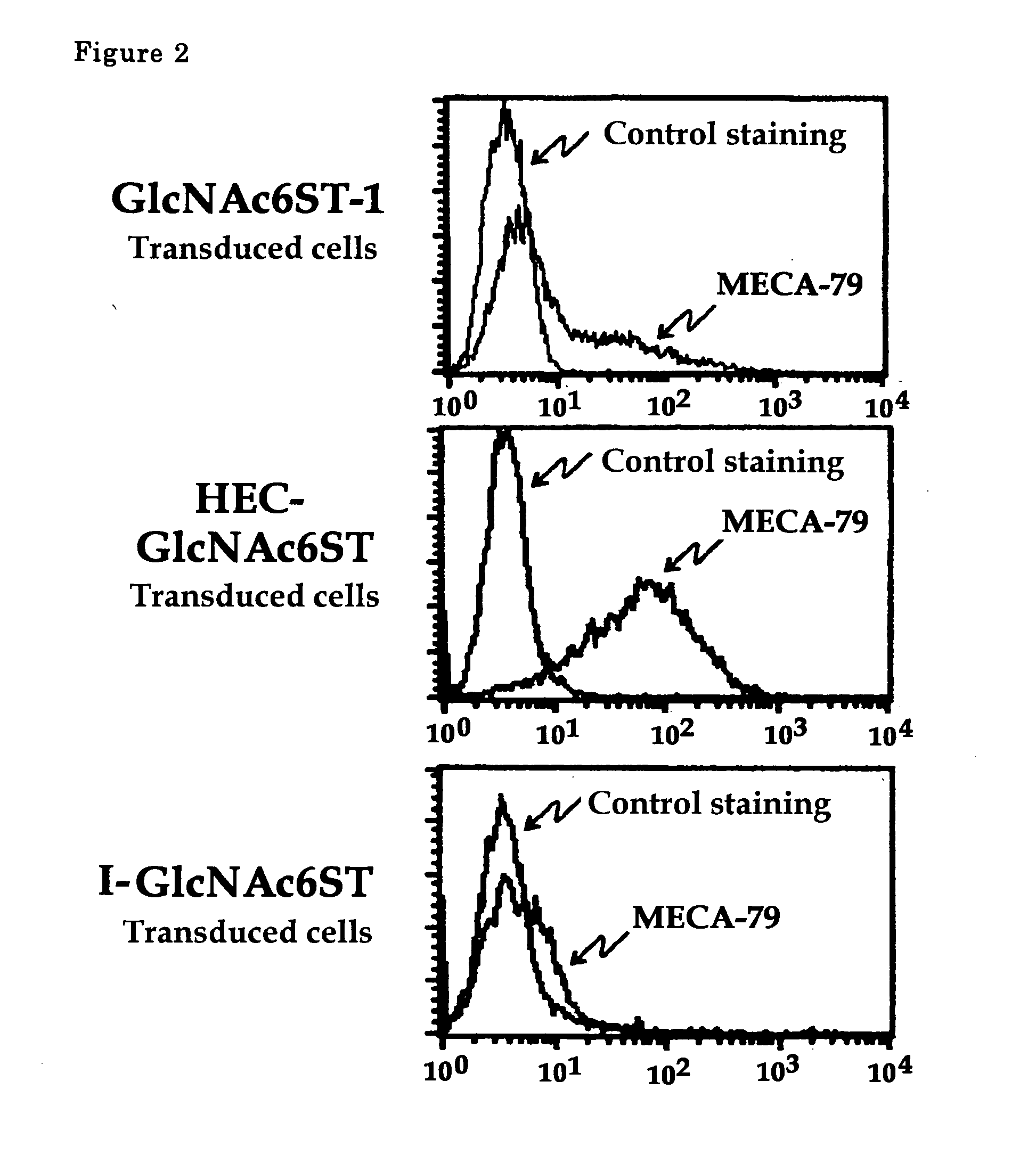
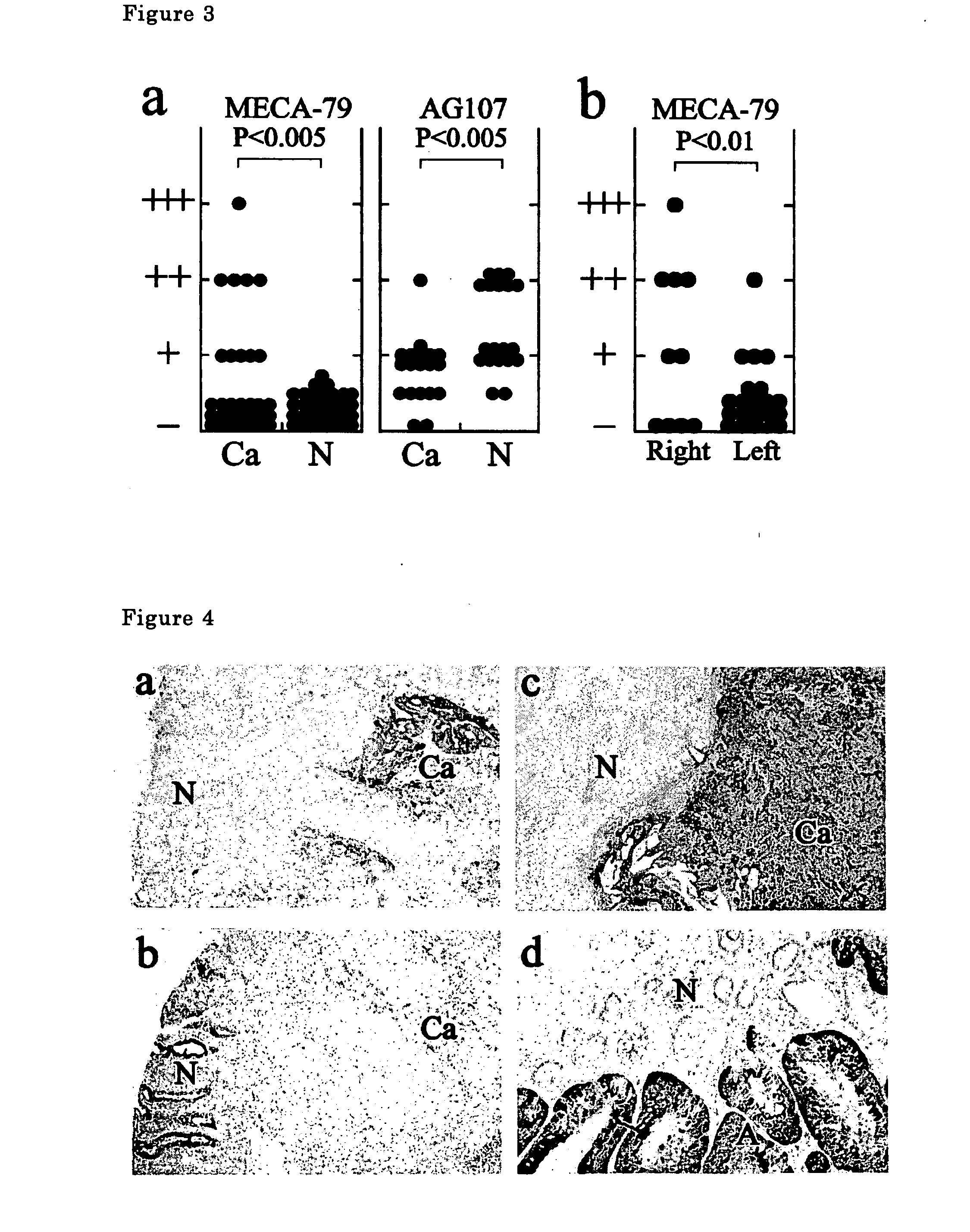
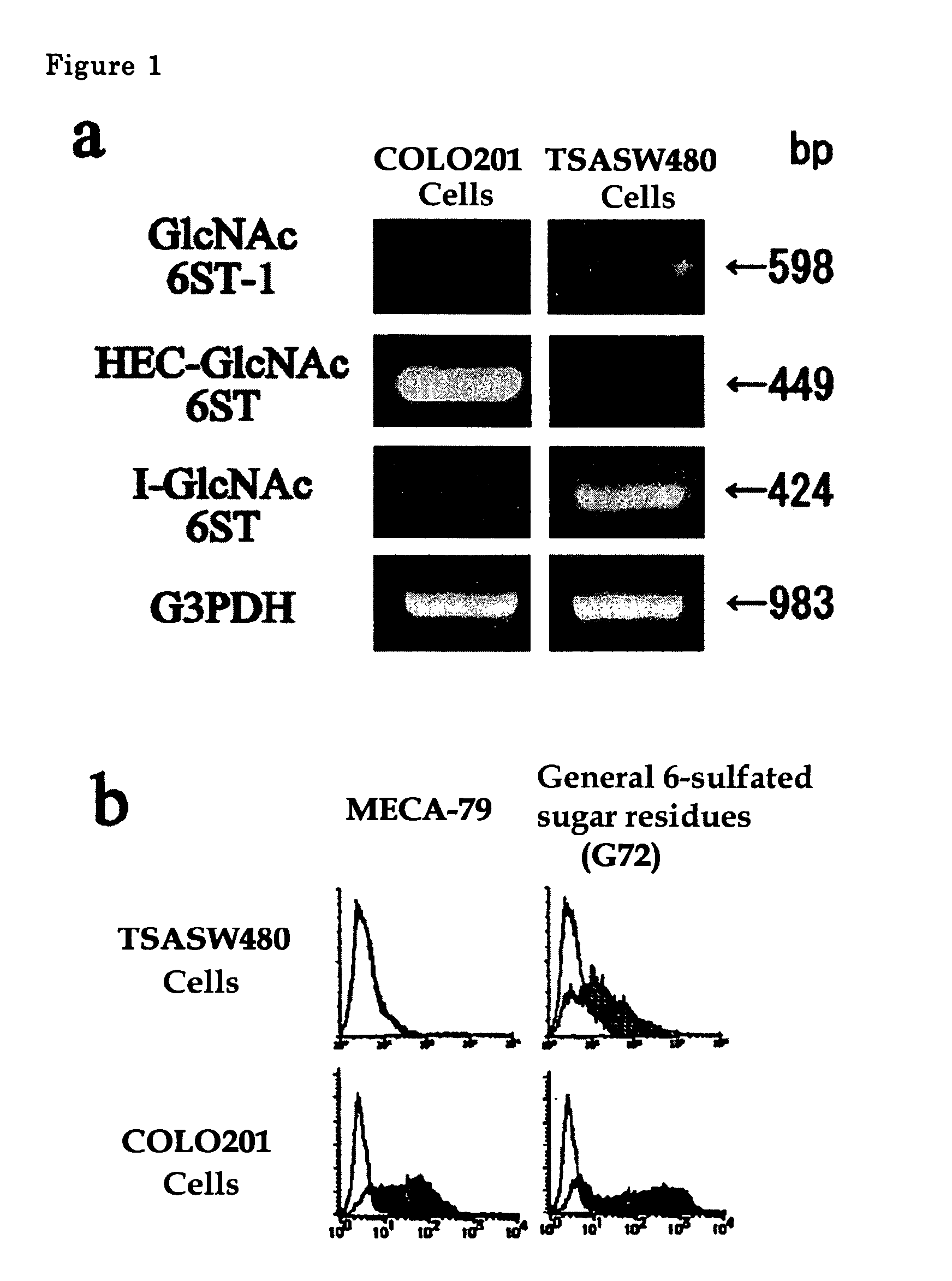
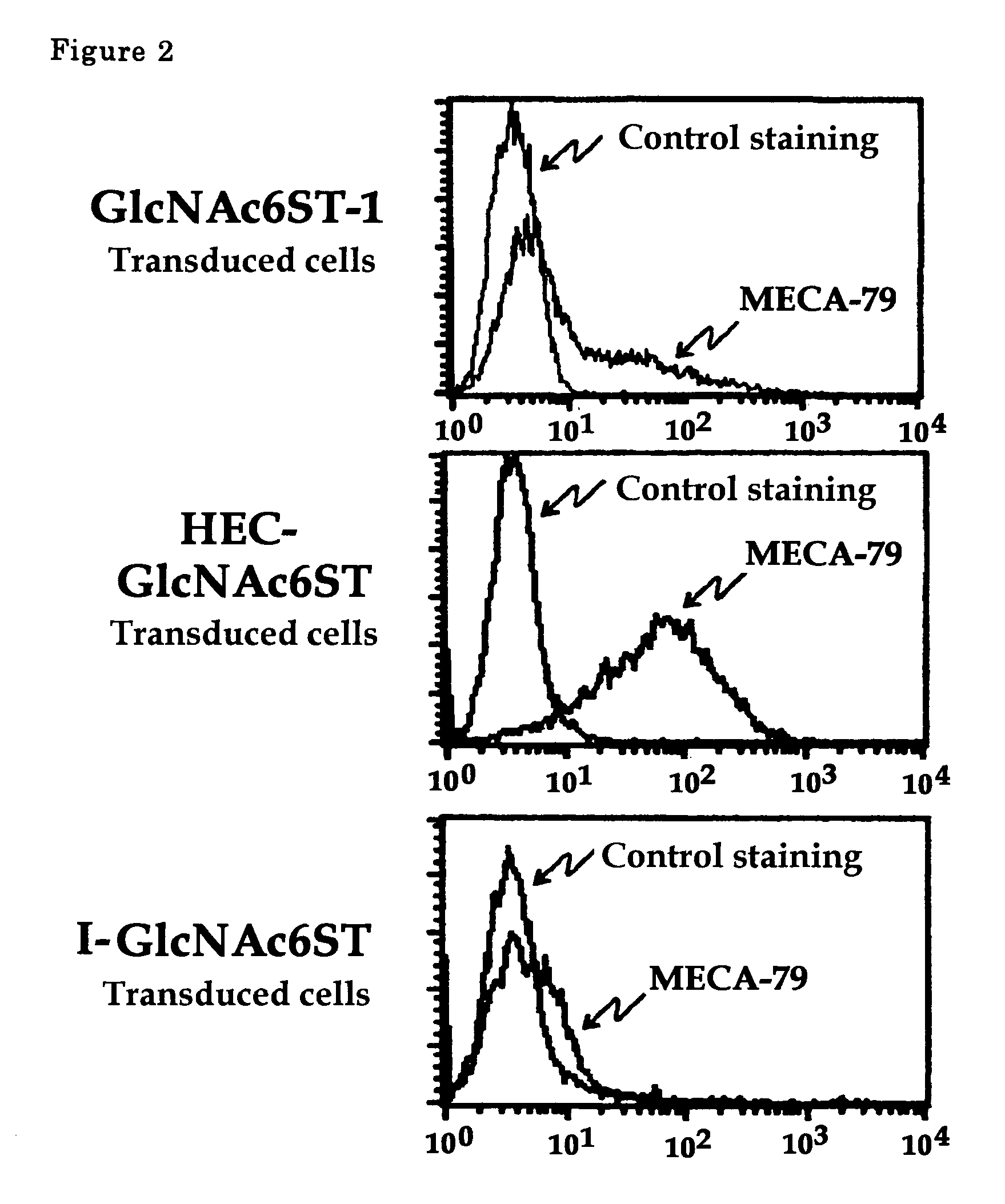
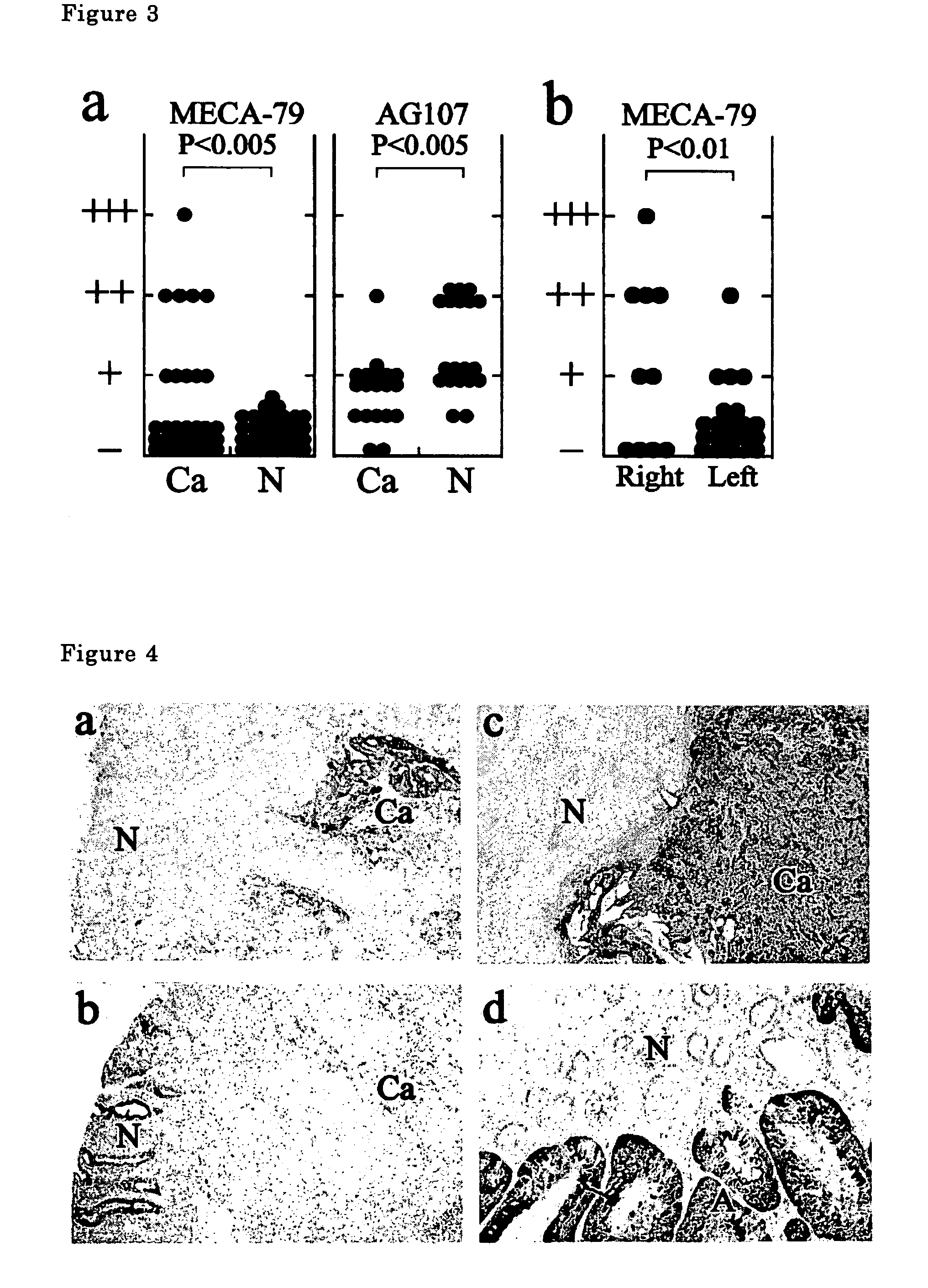
The present invention provides a method for examining colorectal cancer and colorectal adenoma, which enables to detect colorectal cancer patients and patients at high risk of colorectal cancer at a high probability and is useful for diagnosis of colorectal cancer and colorectal adenoma, and provides the examination reagents thereof. The present inventors discovered that there are significant differences in the distribution of GlcNAc-6-sulfotransferase isozymes, sulfation enzymes of sugar residues, among non-cancer colorectal tissues, colorectal cancer tissues and colorectal adenoma tissues. Furthermore the inventors applied the discovery to diagnosis and found that colorectal cancers and adenomas are detected specifically by assaying a definite range of GlcNAc-6-sulfated sugar residues in tissues from patients or feces samples. MECA-79 antibody (Pharmingen, catalog No. 09961D, Distributor: Becton Dickinson), reacting with GlcNAc-6-sulfated sugar residues, which are produced specifically by the enzyme present in colorectal cancer and colorectal adenoma tissues could be used for the examination of colorectal cancers and colorectal adenomas.
Owner:JAPAN SCI & TECH CORP +2
Biomarkers for detecting colorectal cancer or adenoma and methods thereof
ActiveCN113711044AMechanical/radiation/invasive therapiesComponent separationSerum samplesDiagnostic biomarker
A group of diagnostic biomarkers usable for diagnosis of colorectal cancer or colorectal adenoma. A method for detecting colorectal cancer or colorectal adenoma using the group of diagnostic biomarkers is also provided. For example, the method is a non-invasive approach that may utilize serum samples for detecting colorectal cancer. Moreover, the method for detecting colorectal cancer may detect colorectal cancer of different stages (e.g., pre-cancer stage, early stage, middle stage, late stage).
Owner:中精普康(北京)医药科技有限公司
Humanized intestinal cancer precancerous lesion immortalized epithelial cell line and construction method and application thereof
ActiveCN111172114AProliferative activity in vitroValue-added activity did not change significantlyGastrointestinal cellsGenetically modified cellsOncologyViral vector
The invention relates to a humanized intestinal cancer precancerous lesion immortalized epithelial cell line and a construction method and application thereof. The construction method comprises the following steps: firstly, transfecting primarily separated human colorectal adenoma polyp epithelial cells by using an SV40 overexpression lentiviral vector, then performing screening by using puromycin, and finally amplifying the screened cells to obtain the humanized intestinal cancer precancerous lesion immortalized epithelial cell line. The humanized intestinal cancer precancerous lesion immortalized epithelial cell line constructed by the invention overcomes the problems that conventional adenoma polyp cannot be subjected to passage in vitro, is low in cell proliferation and poor in cell activity, and cannot meet the cell passage requirement. According to the invention, the humanized intestinal cancer precancerous lesion immortalized epithelial cell line is established for the first time, an important cell experiment tool is provided for developing an in-vitro experiment of intestinal cancer precancerous lesions, and preclinical researches such as new drug screening and drug effectcomponents are also convenient to develop.
Owner:YUEYANG INTEGRATED TRADITIONAL CHINESE & WESTERN MEDICINE HOSPITAL SHANGHAI UNIV OF CHINESE TRADITIONAL MEDICINE
Method of examining colon cancer and colon adenoma
The present invention provides a method for examining colorectal cancer and colorectal adenoma, which enables to detect colorectal cancer patients and patients at high risk of colorectal cancer at a high probability and is useful for diagnosis of colorectal cancer and colorectal adenoma, and provides the examination reagents thereof. The present inventors discovered that there are significant differences in the distribution of GlcNAc-6-sulfotransferase isozymes, sulfation enzymes of sugar residues, among non-cancer colorectal tissues, colorectal cancer tissues and colorectal adenoma tissues. Furthermore the inventors applied the discovery to diagnosis and found that colorectal cancers and adenomas are detected specifically by assaying a definite range of GlcNAc-6-sulfated sugar residues in tissues from patients or feces samples. MECA-79 antibody (Pharmingen, catalog No. 09961D, Distributor: Becton Dickinson), reacting with GlcNAc-6-sulfated sugar residues, which are produced specifically by the enzyme present in colorectal cancer and colorectal adenoma tissues could be used for the examination of colorectal cancers and colorectal adenomas.
Owner:JAPAN SCI & TECH CORP +2
Method of diagnosing neoplasms
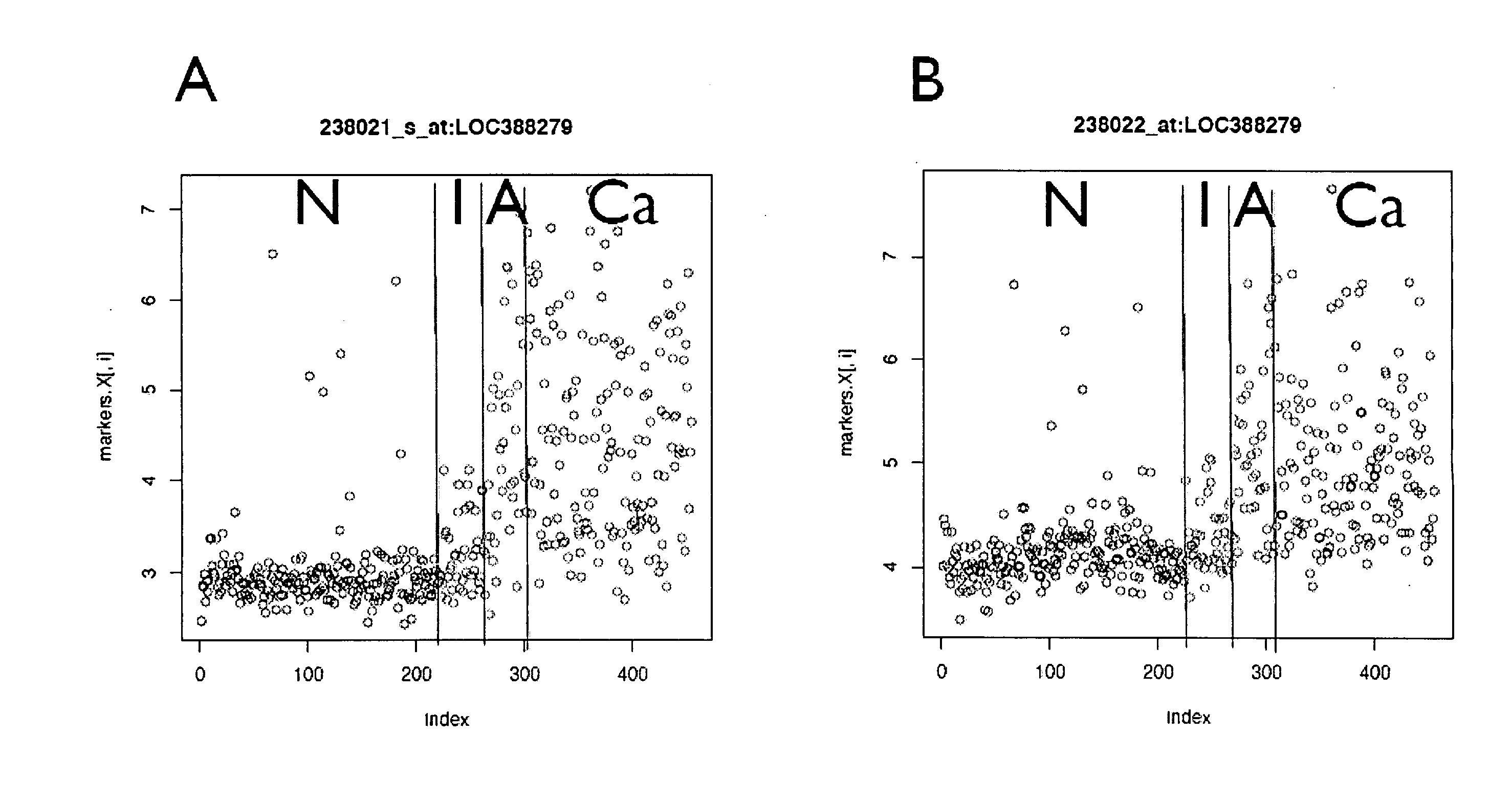
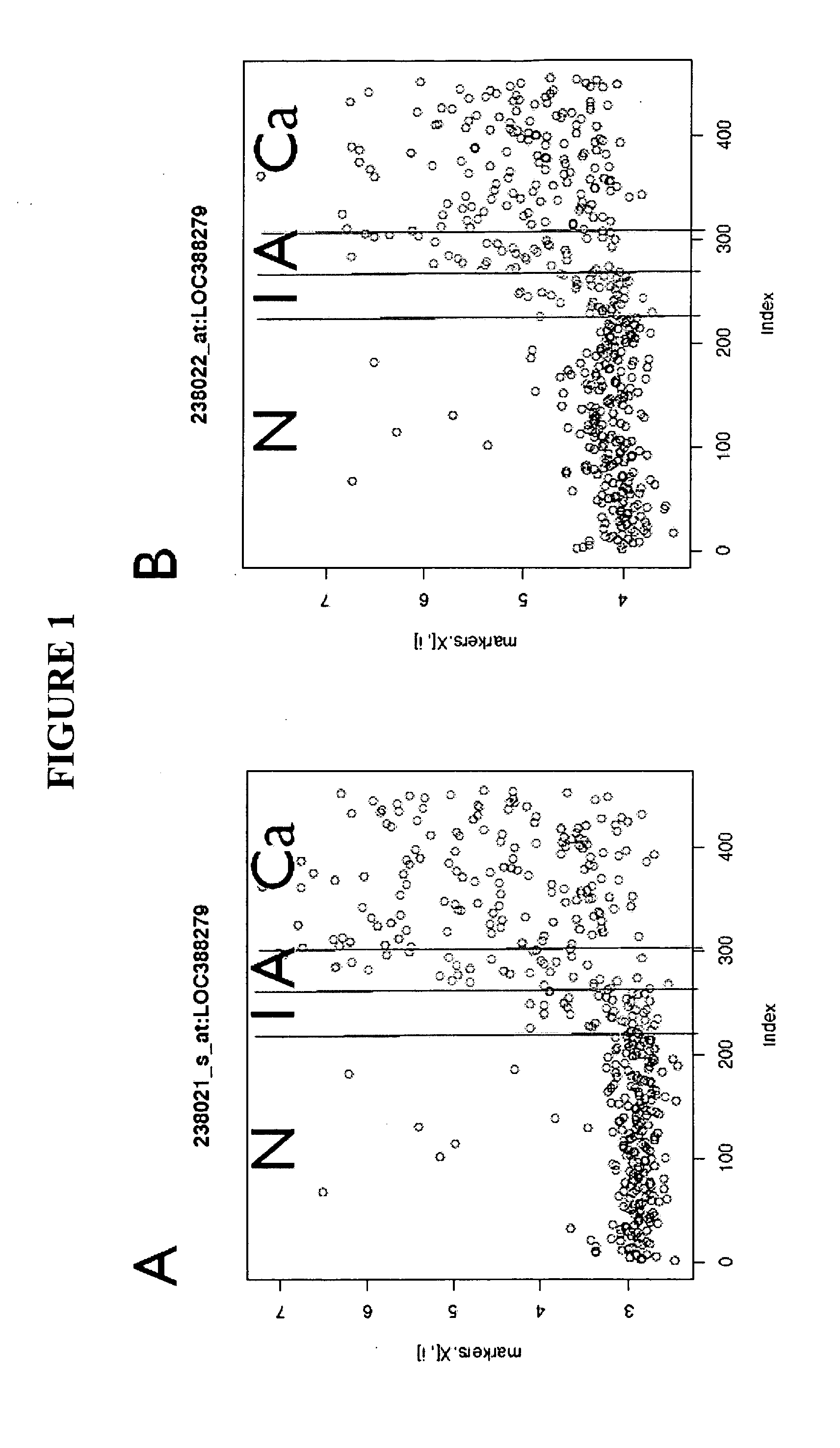
The present invention relates generally to a nucleic acid molecule, the RNA and protein expression profiles of which are indicative of the onset, predisposition to the onset and / or progression of a large intestine neoplasm. More particularly, the present invention is directed to a nucleic acid molecule, the expression profiles of which are indicative of the onset and / or progression of a colorectal neoplasm, such as an adenoma or an adenocarcinoma. The expression profiles of the present invention are useful in a range of applications including, but not limited to, those relating to the diagnosis and / or monitoring of colorectal neoplasms, such as colorectal adenomas and adenocarcinomas. Accordingly, in a related aspect the present invention is directed to a method of screening a subject for the onset, predisposition to the onset and / or progression of a large intestine neoplasm by screening for modulation in the expression profile of said nucleic acid molecule markers.
Owner:CLINICAL GENOMICS PTY LTD +1
Leveraging sequence-based fecal microbial community survey data to identify a composite biomarker for colorectal cancer
InactiveUS20200011873A1Risk of developMicrobiological testing/measurementSequence analysisFecesIntestinal microorganisms
The present disclosure provides fecal microbial markers for diagnosing colorectal cancer and colorectal adenoma. The present disclosure also provides methods for diagnosing colorectal cancer and colorectal adenoma using these intestinal microbial markers.
Owner:SECOND GENOME +1
Method of diagnosing neoplasms
InactiveUS20140155280A1Microbiological testing/measurementLibrary screeningNeoplasmColorectal neoplasm
The present invention relates generally to a method for screening a subject for the onset, predisposition to the onset and / or progression of a colorectal neoplasm by screening for modulation in the level of expression of one or more nucleic acid markers. More particularly, the present invention provides a method for screening a subject for the onset, predisposition to the onset and / or progression of a colorectal neoplasm by screening for modulation in the level of expression of one or more gene markers in membranous microvesicles. The expression profiles of the present invention are useful in a range of applications including, but not limited to, those relating to the diagnosing and / or monitoring of colorectal neoplasms, such as colorectal adenoma and adenocarcinomas.
Owner:CLINICAL GENOMICS PTY LTD +1
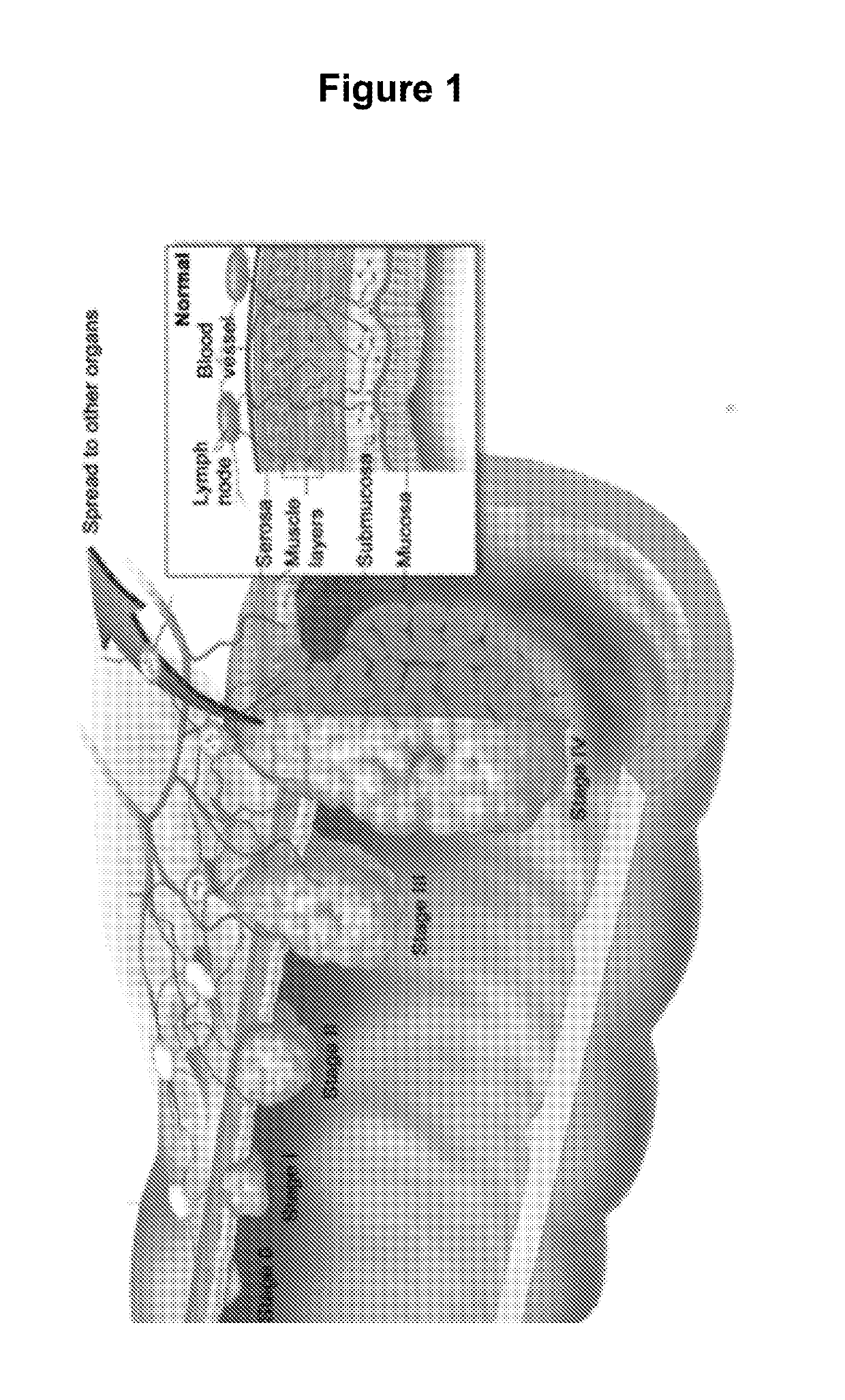
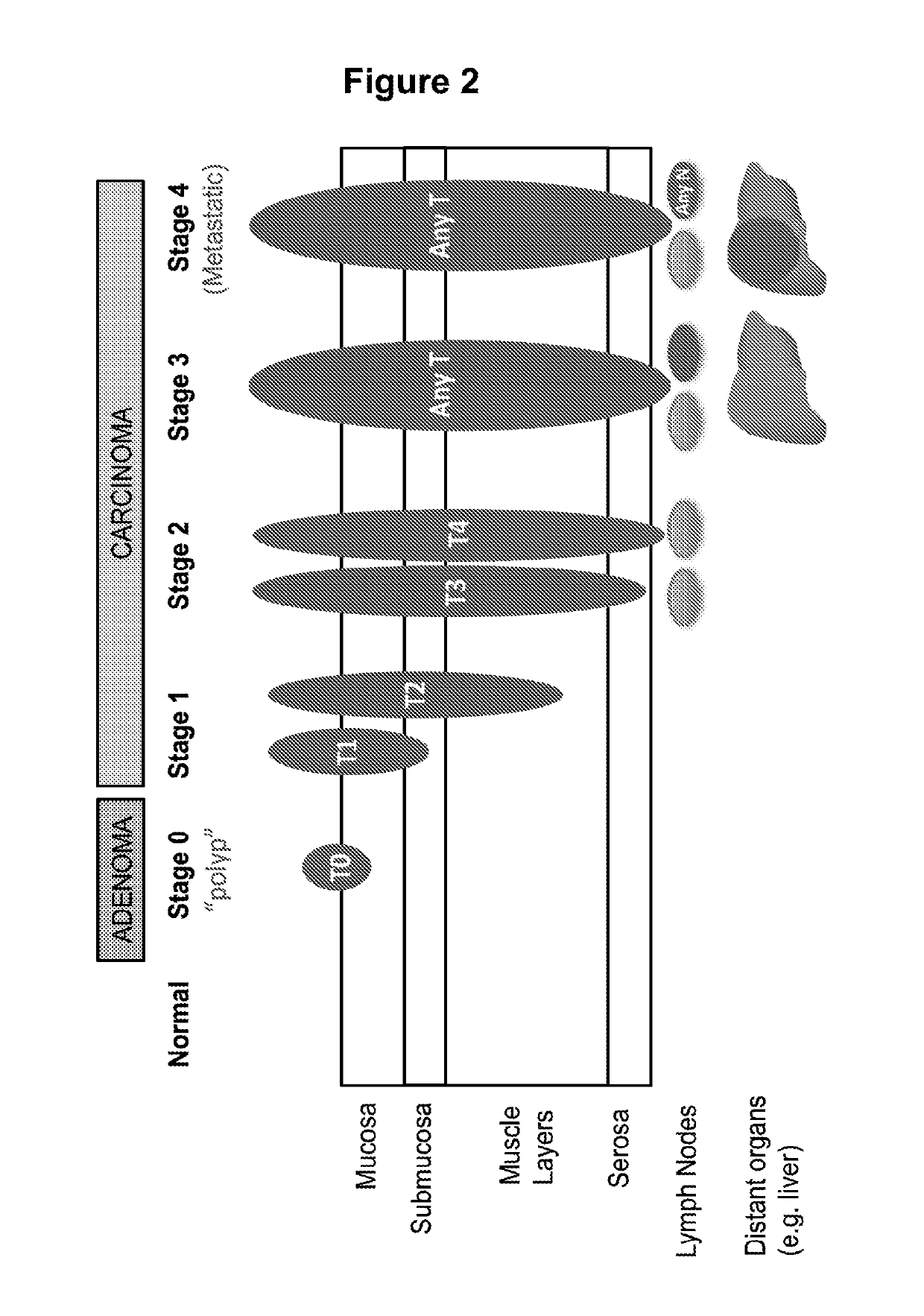
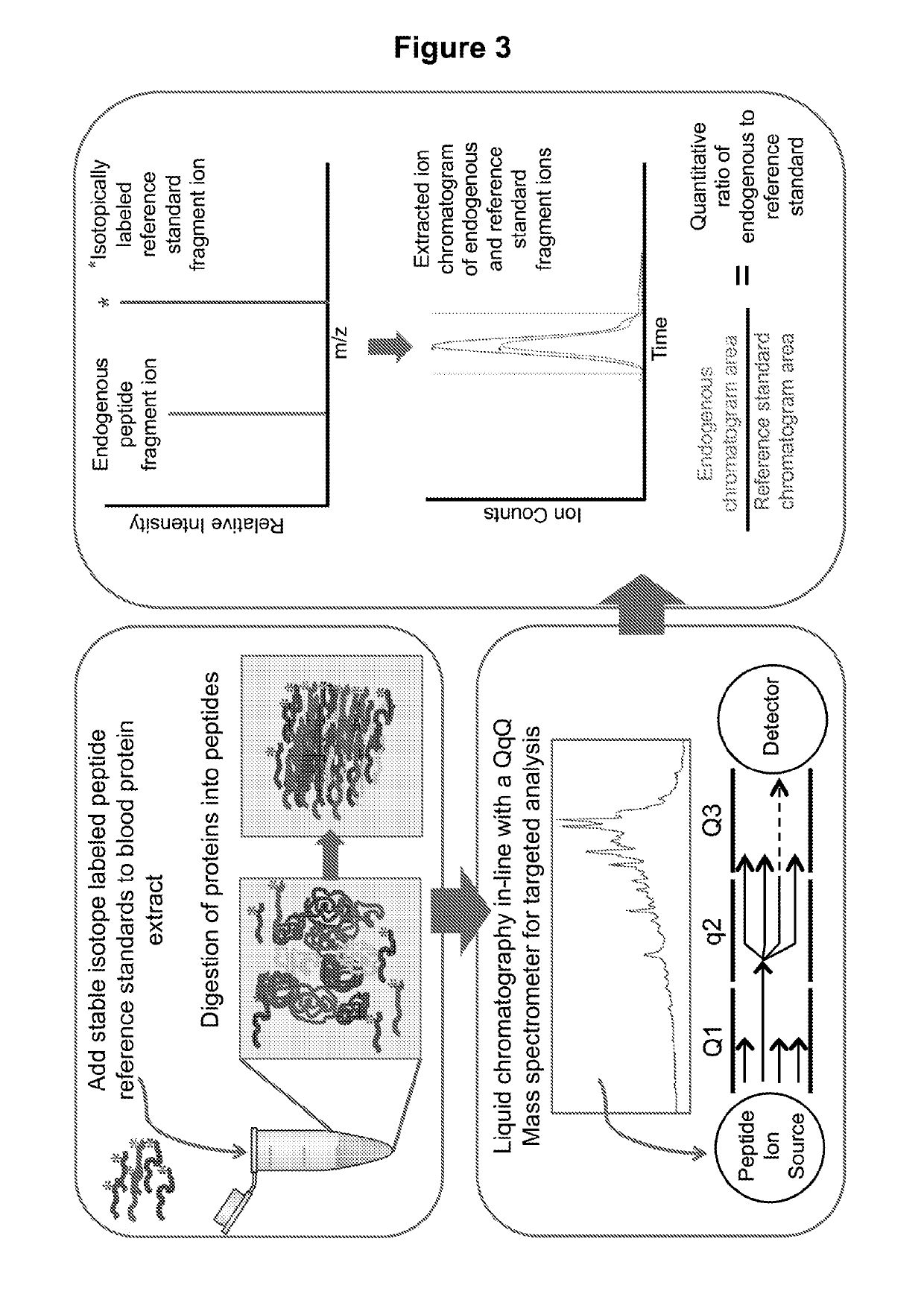
Methods for Detection, Staging, and Surveillance of Colorectal Adenomas and Carcinomas
Owner:WISCONSIN ALUMNI RES FOUND
Preparation and application of kit used for testing content of lipid molecules in human blood sample and diagnosing colorectal adenomas
The invention provides a lipid molecule combination used as a biomarker. The lipid molecule combination is prepared into a kit, human blood is used as the sample, the content of lipid marking molecules selected in the kit is tested and analyzed comprehensively, and the possibility of colorectal adenomas of the tested unit is determined according to the analysis result so that colorectal adenomas is detected early and a patient is diagnosed and treated in time.
Owner:桑建利
Kit for screening colorectal adenomas (CRA)
The invention discloses a kit for screening colorectal adenomas (CRA). The kit for screening or screening CRA in an aided manner comprises a device for detecting the contents of phospholipids and a comparison card established by the contents of phospholipids, wherein the phospholipids include 16:0SM, 18:0SM, 14:0LPC, 16:0LPC, 18:2LPC, 18:1LPC, 18:0LPC, 20:4LPC, 20:0LPC, 22:6LPC and 22:0LPC. A molecular model for distinguishing healthy controls from CRA patients is established by taking saturated LPC, 20:4LPC and SM as markers, and the sensitivity and specificity of the model in distinguishing the CRA are respectively 89% and 80%.
Owner:BEIJING NORMAL UNIVERSITY
Pharmaceutical composition containing medicinal calcium salt for early preventing colorectal adenoma or colorectal cancer
InactiveCN102085217AInhibit malignant transformationInhibition of proliferative activityInorganic phosphorous active ingredientsDigestive systemWilms' tumorFhit gene
The invention discloses a pharmaceutical composition containing medicinal calcium salt for early preventing colorectal adenoma or colorectal cancer. The pharmaceutical composition contains sodium butyrate and medicinal calcium salt, wherein the mass ratio of the sodium butyrate to elemental calcium in the pharmaceutical composition is (2.47-4.95):1. The pharmaceutical composition disclosed by the invention can reduce cancer genes related to the colorectal cancer and can increase related cancer suppressor genes and signal passages to suppress colorectal cancer cell proliferation and migration capabilities and retard cell growth cycle, thereby achieving the purposes of suppressing the occurrence of the colorectal cancer, lowering the incidence rate of the colorectal cancer of mice, and reducing the tumor volume.
Owner:RENJI HOSPITAL AFFILIATED TO SHANGHAI JIAO TONG UNIV SCHOOL OF MEDICINE
Traditional Chinese medicine composition and application thereof
ActiveCN113713057AImprove immunityEnhance anti-inflammatory and anti-oxidantDigestive systemImmunological disordersLicorice rootsBULK ACTIVE INGREDIENT
The invention relates to a traditional Chinese medicine composition for preventing and treating colorectal adenoma, colorectal adenocarcinoma and relapse after endoscopic resection of colorectal adenoma. The traditional Chinese medicine composition is characterized in that the traditional Chinese medicine composition is prepared from the following main active ingredients in parts by weight: 1-300 parts of poria cocos, 1-300 parts of Chinese yam, 1-300 parts of coix seed, 1-300 parts of Chinese actinidia root, 1-250 parts of duchesnea indica, 1-250 parts of sargentgloryvine stem, 1-250 parts of elecampane and 1-120 parts of honey-fried licorice root. The invention further provides application of the traditional Chinese medicine composition in preparation of medicines for improving immunity of organisms and application of the traditional Chinese medicine composition in preparation of medicines for preventing and treating colorectal adenoma, colorectal adenocarcinoma and relapse after endoscopic resection of the colorectal adenoma.
Owner:段鲜红
Exosome RNA molecular marker combination for diagnosis of colorectal adenoma and application thereof
ActiveCN111455053AImprove complianceHigh sensitivityMicrobiological testing/measurementDNA/RNA fragmentationExosomeRNA marker
The invention relates to the field of biological detection, in particular to molecular detection of organisms, more particularly to a group of exosome RNA markers associated with colorectal adenoma and an application thereof.
Owner:BEIJING FRIENDSHIP HOSPITAL CAPITAL MEDICAL UNIV
Plasma marker combination for early diagnosis or prognosis prediction of colorectal cancer
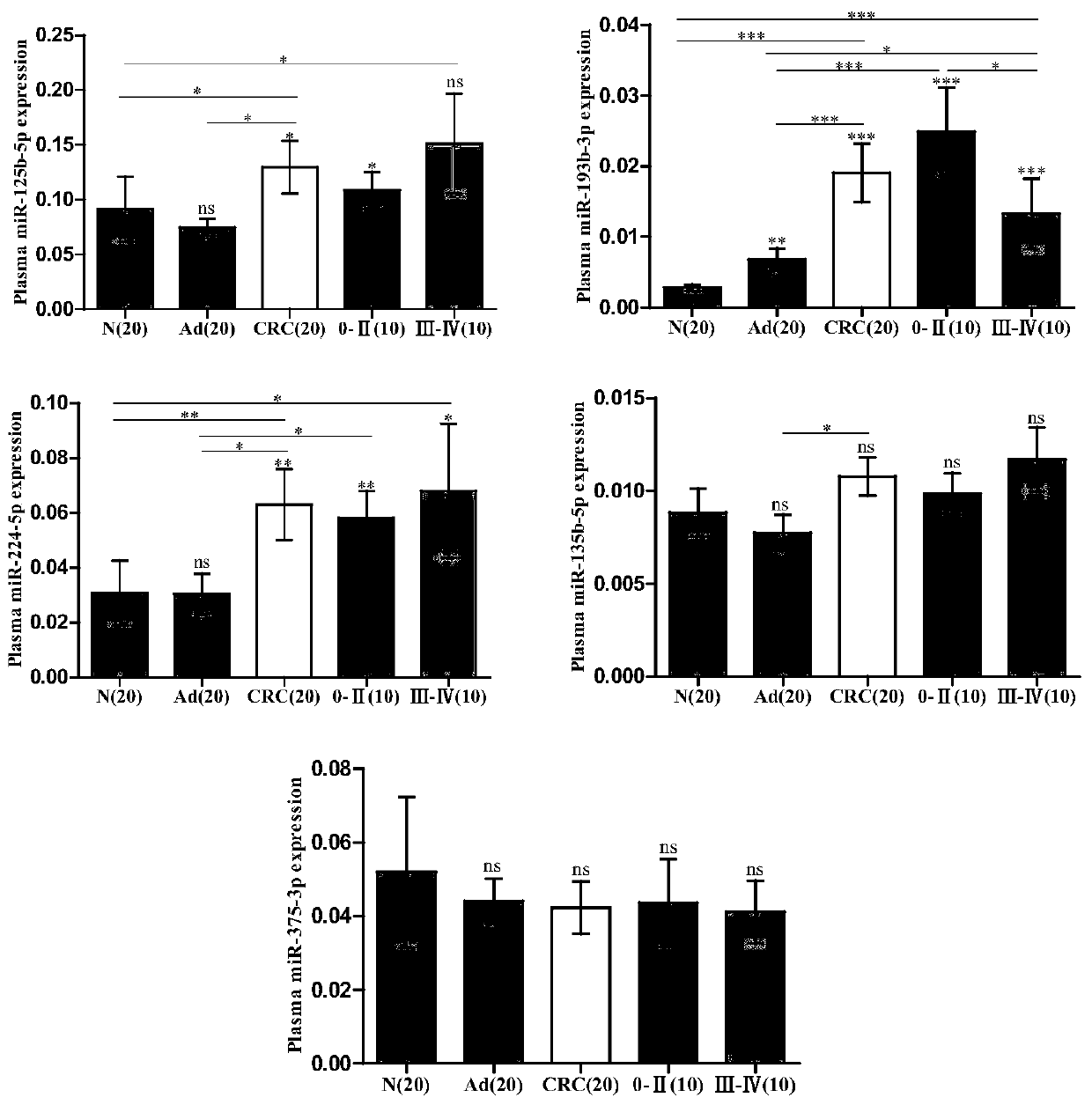
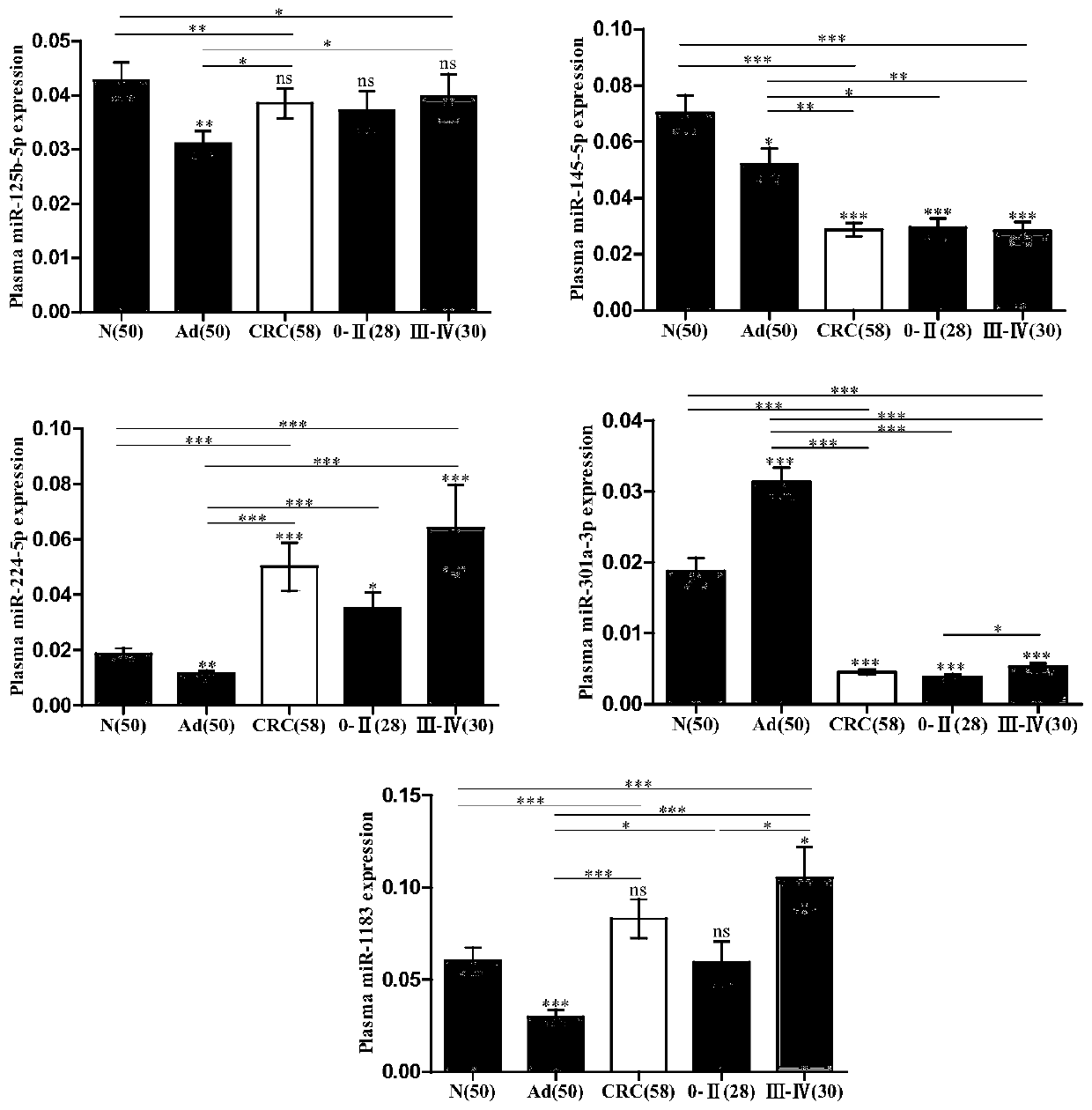
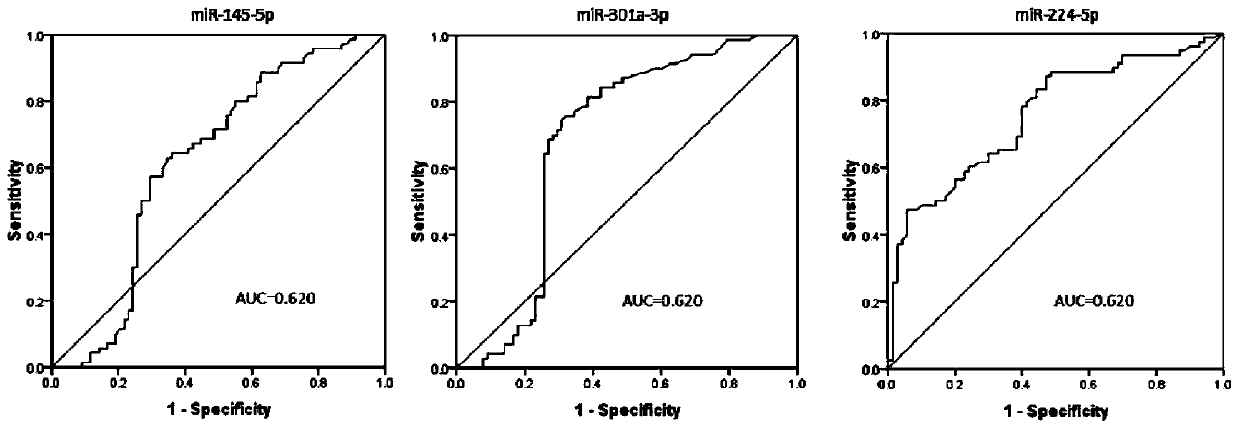
The invention discloses a plasma marker combination for early diagnosis or prognosis prediction of colorectal cancer. Specifically, the present invention obtains miRNA which is obviously abnormally expressed in colorectal cancer plasma, namely miR-224-5p, miR-301a-3p, miR-145-5p, miR-193b-3p and miR-1183. The invention finds that the plasma miRNA combination can be used as a marker for early diagnosis or prognosis judgment of colorectal cancer by researching the expression difference of the plasma miRNA in patients with colorectal cancer and colorectal adenoma at various stages and healthy people.
Owner:THE THIRD AFFILIATED HOSPITAL OF GUANGZHOU MEDICAL UNIVERSITY +1
Methods and tools for discriminating colorectal adenomas and adenocarcinomas
The present invention relates to the differential expression of genes in colorectal adenoma and adenocarcinoma cells and their correlation with chromosomal aberrations. The present invention provides tools for detecting chromosomal aberrations linked to progression of adenomas into adenocarcinoma cells. The present invention discloses methods and tools for in vivo and in vitro diagnosis of colorectal tumours.
Owner:基督教高等教育科学研究及病人护理协会
Acf detection method
InactiveUS20140287412A1Improve expression levelMicrobiological testing/measurementPeptide preparation methodsLarge intestineMolecular level
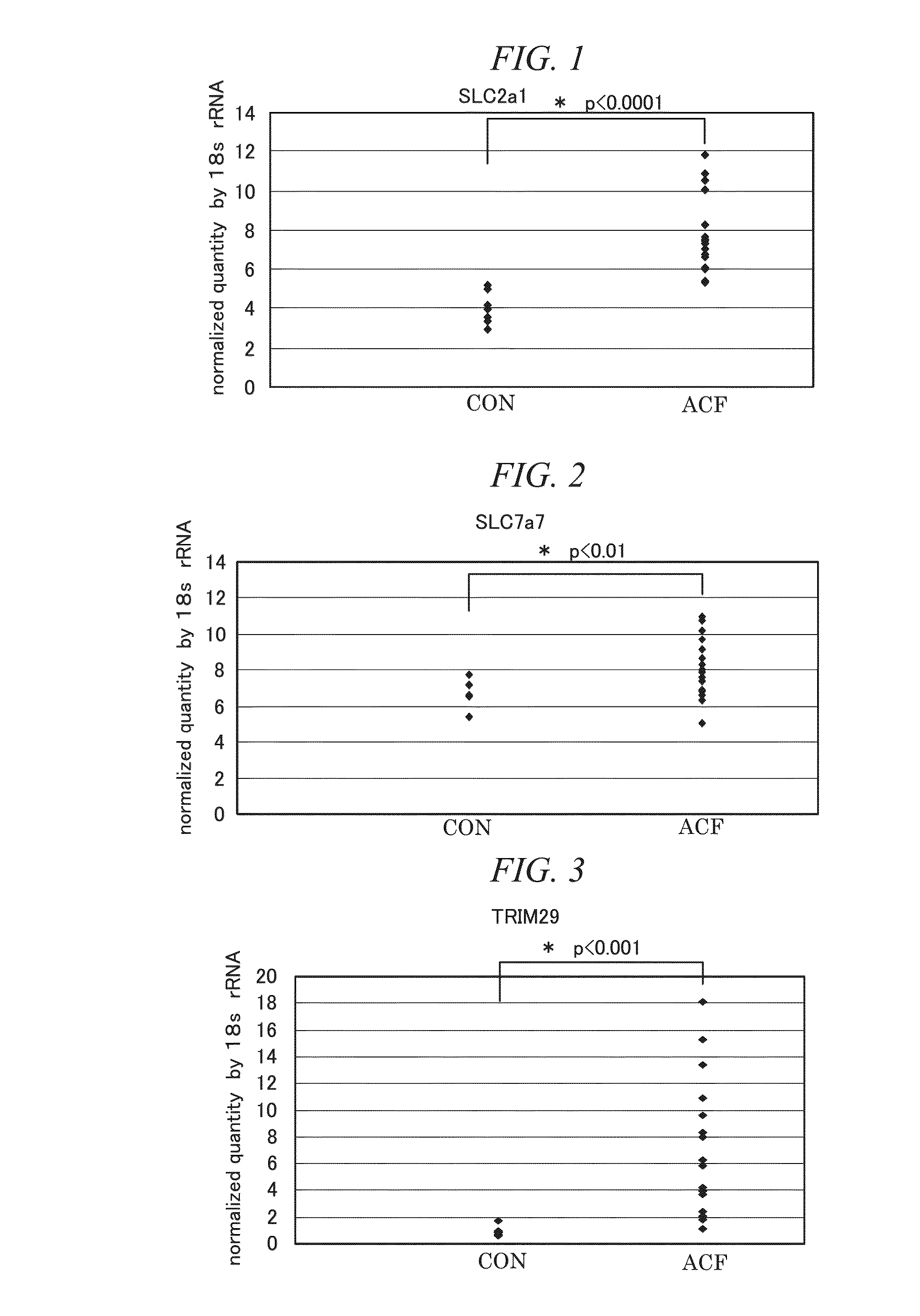
The present invention provides a method for detecting ACF by analyzing a test region of large intestine tissue at the molecular level. Namely, the present invention relates to a method for detecting aberrant crypt foci (ACF) that comprises detecting an ACF detection marker in a test region of large intestine tissue, by using one or more types of molecules for which ACF-specific expression increases as the ACF detection marker, the molecule being selected from the group consisting of SLC2a1, and SLC7a7; an ACF detection marker for detecting the ACF in human-derived large intestine tissue, that is SLC2a1, or SLC7a7; and, a method for evaluating risk of colorectal cancer and colorectal adenoma in subjects based on the results of detecting ACF in a test region of large intestine tissue of the subjects using the aforementioned ACF detection method.
Owner:OLYMPUS CORP +1
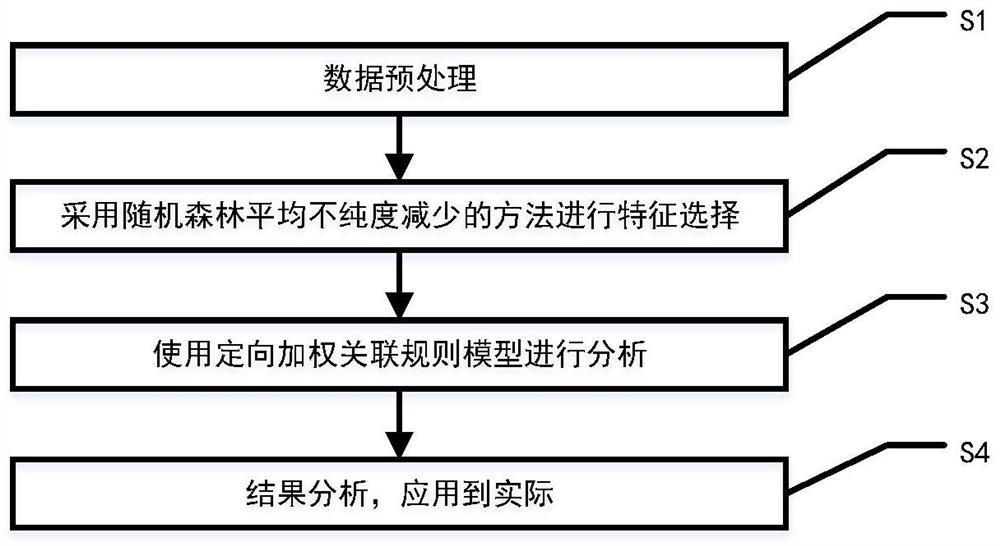
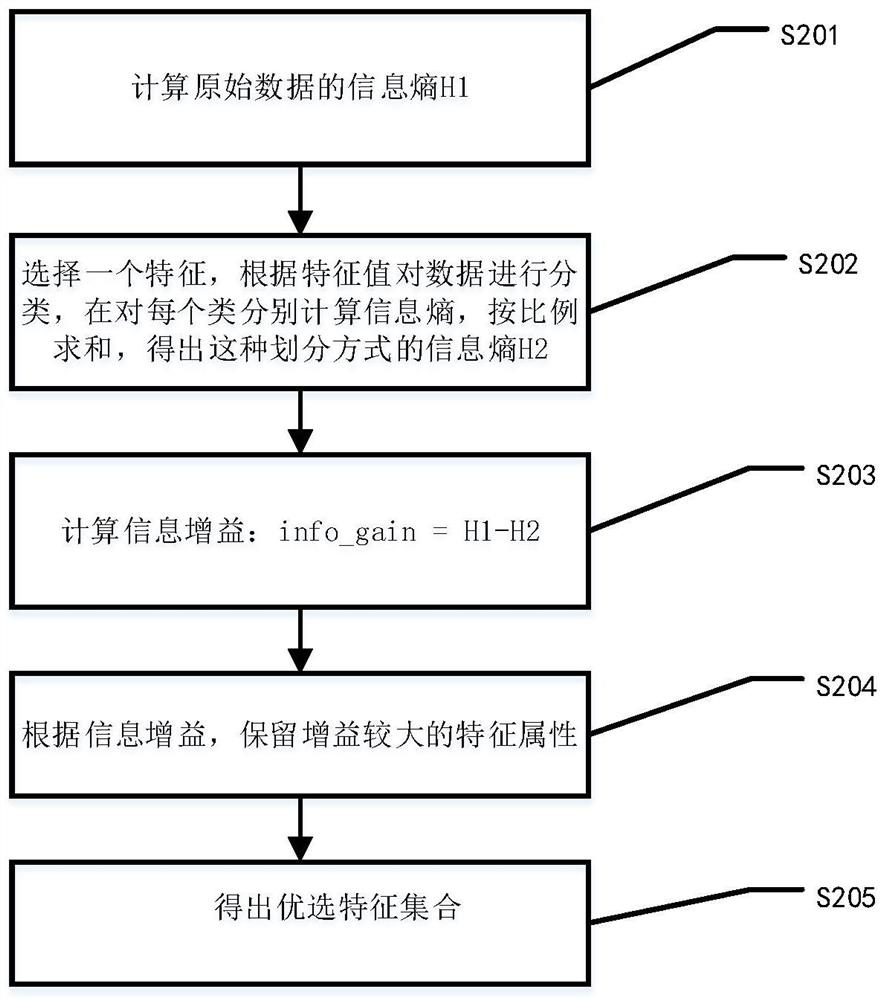
Method for screening risk factors of diffuse colorectal adenoma based on directional weighted association rule model
The invention discloses a method for screening risk factors of diffuse colorectal adenoma based on a directional weighted association rule model, and belongs to the field of data mining. The method comprises the following steps: firstly, preprocessing data; secondly, performing feature extraction by adopting a feature selection method for reducing the average impure degree of the random forest, and determining an optimal division node by utilizing information gain to obtain an optimal feature set; then, inputting the optimal feature set into a directional weighted association rule model to generate a strong association rule; and finally, bringing the risk factors contained in the strong association rule into a risk factor set, and communicating with experts. Compared with the prior art, the method mainly provides a directional weighted association rule model to screen the risk factors of colorectal adenoma, affirms the significance of living dietary habit factors in the etiology of colorectal adenoma, finds out unfound high-risk factors in the previous research, and provides a set of method worthy of reference for finding out the risk factors of colorectal adenoma.
Owner:SHANGHAI MARITIME UNIVERSITY
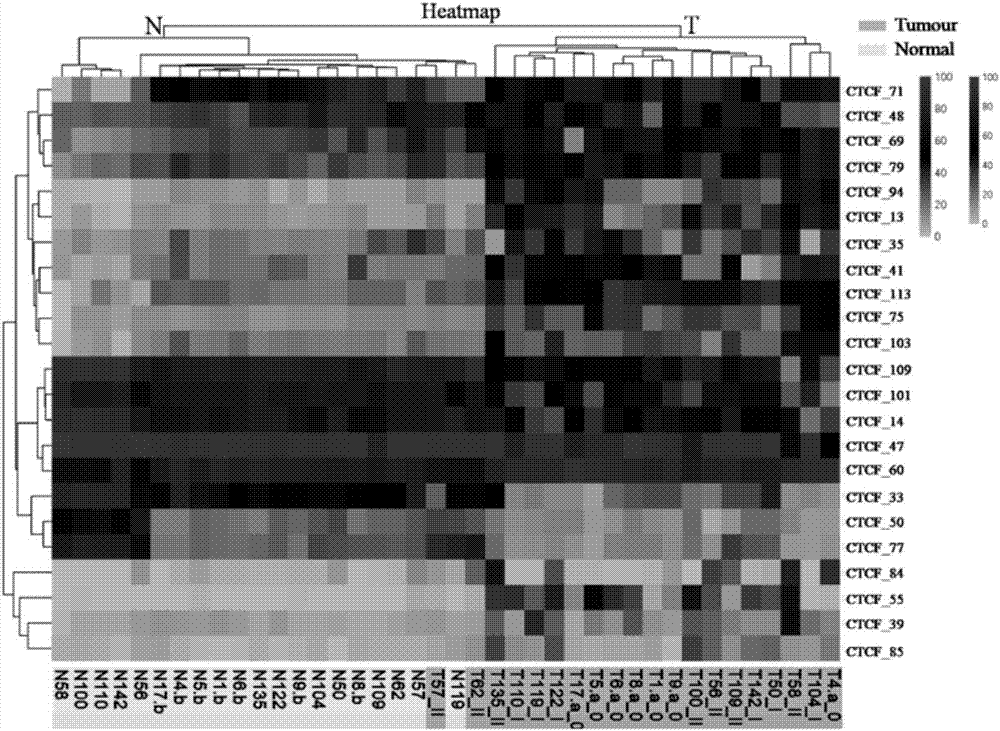
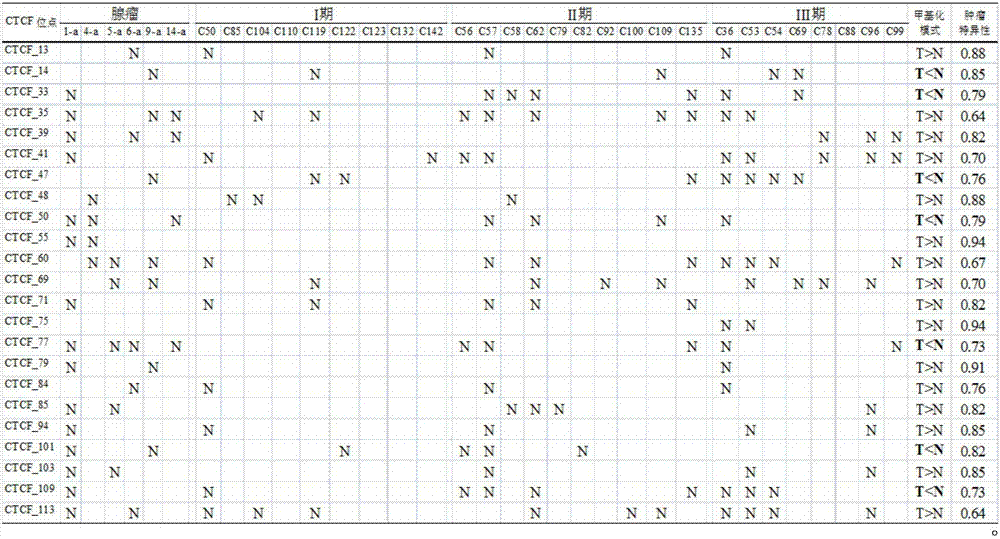
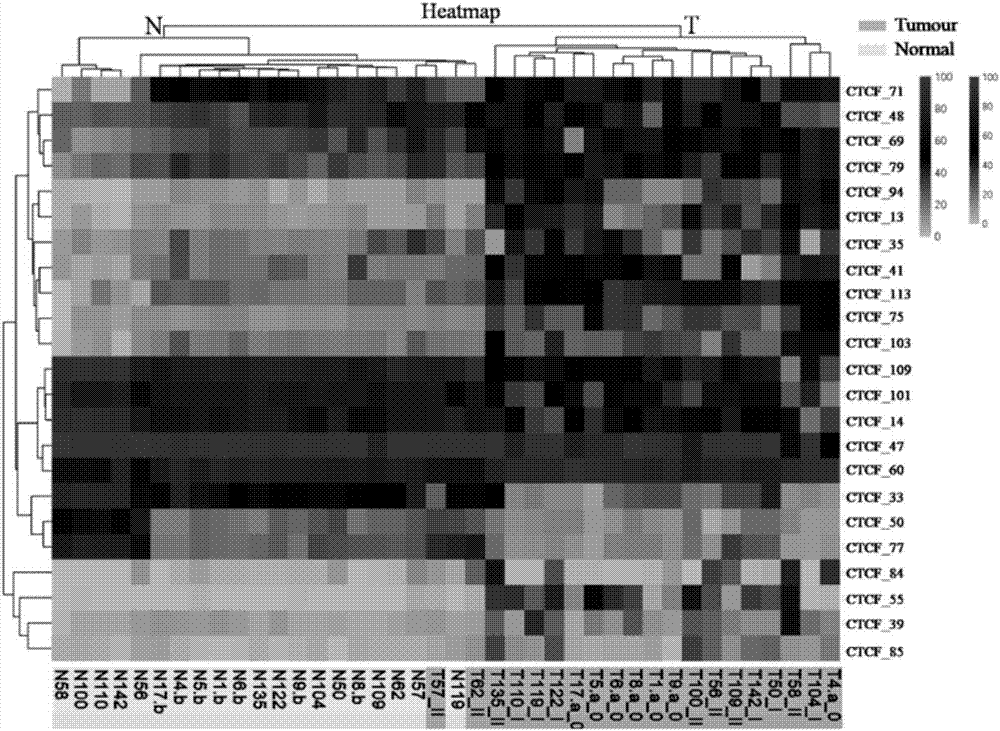
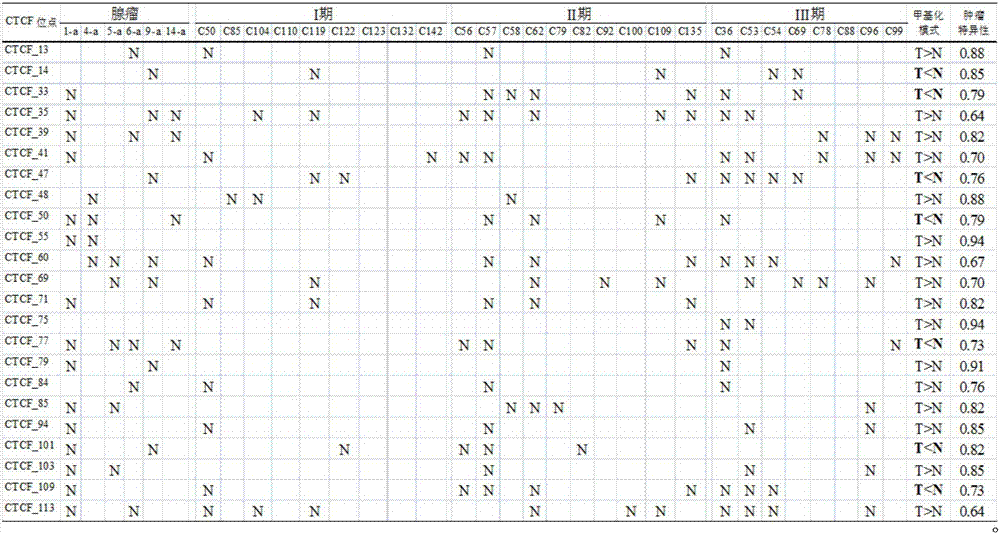
Application of DNA binding site CTCF_55 of multifunctional transcriptional regulation factor CTCF
The invention discloses novel application of a DNA binding site CTCF_55 of a multifunctional transcriptional regulation factor CTCF, and particularly relates to application of the DNA binding site CTCF_55 in preparation of a kit for early diagnosis of a colorectal tumor. The nucleotide of the binding site is shown as SEQ ID NO: 1. The accuracy of the CTCF binding site provided by the invention for detecting colorectal adenomas or a colorectal cancer is obviously higher than that of a reported DNA methylated molecular marker; furthermore, the DNA binding site of the CTCF may be a biological marker for monitoring the tumor treatment effect, estimating the prognosis effect and tumor molecular parting (such as CIMP parting). In conclusion, the invention provides a new idea for searching a more effective molecular marker for early diagnosis of the tumor, monitoring of the treatment effect, estimation of the prognosis effect and the molecular parting, and the application has important significance for prevention and treatment of the tumor.
Owner:FIRST AFFILIATED HOSPITAL OF KUNMING MEDICAL UNIV
In vitro method for identifying colorectal adenomas or colorectal cancer
InactiveUS20190316212A1Accurately screening and diagnosing subjectsMicrobiological testing/measurementDiseaseColo-rectal cancer
The present invention refers to an in vitro method for identifying patients at risk of suffering from colorectal cancer and / or colorectal adenomas, preferably advanced colorectal adenomas, based on measuring the expression profile or level of some miRNAs, e.g. miR-15b, which are up-regulated or over-expressed in patients suffering from said diseases.
Owner:ADVANCED MARKER DISCOVERY SL
Nucleic acid markers for use in determining predisposition to neoplasm and/or adenoma
The present invention relates generally to novel nucleic acid molecules, the levels and / or patterns of expression of which are indicative of the onset, predisposition to the onset and / or progression of a neoplasm and to derivatives, homologues or analogues of said molecules. More particularly, the present invention is directed to novel nucleic acid molecules, the levels of expression of which are indicative of the onset and / or progression of a gastrointestinal tract neoplasm, such as an adenoma, and to derivatives, homologues or analogues of said molecules. The present invention is further directed to isolated proteins encoded thereby and to derivatives, homologues, analogues, chemical equivalents and mimetics thereof. The molecules of the present invention are useful in a range of prophylactic, therapeutic and / or diagnostic applications including, but not limited to, those relating to the diagnosis and / or treatment of colorectal neoplasms such as colorectal adenomas. In a related aspect, the present invention is directed to a method of screening a subject for the onset, predisposition to the onset and / or progression of a neoplasm by screening for modulation in the level of expression of one or more nucleic acid molecule markers.
Owner:CLINICAL GENOMICS PTY LTD
Application of DNA (deoxyribonucleic acid) binding site CTCF_13 of multifunctional transcriptional regulation factor CTCF
InactiveCN107151708AImprove accuracyEffective early diagnosisMicrobiological testing/measurementColorectal adenomaMolecular typing
The invention discloses a novel use of a DNA (deoxyribonucleic acid) binding site CTCF_13 of a multifunctional transcription regulation factor CTCF, i.e. application thereof to preparation of a kit for early diagnosis of colorectal neoplasms. The nucleotide sequence of the binding site is shown as SEQ ID NO:1. The accuracy of the binding site CTCF, provided by the invention, in detection of either colorectal adenomas or the colorectal neoplasms is remarkably higher than that of a reported DNA methylated molecular marker; moreover, the DNA binding site of the CTCF is also likely to become a biomarker for tumor treatment effect monitoring, prognosis effect evaluation and tumor molecular typing (such as CIMP typing). In brief, a novel concept is provided for seeking for a more effective molecular marker for early diagnosis of tumors, treatment effect monitoring, prognosis effect evaluation and molecular typing, and is significant for tumor prevention and treatment.
Owner:FIRST AFFILIATED HOSPITAL OF KUNMING MEDICAL UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com