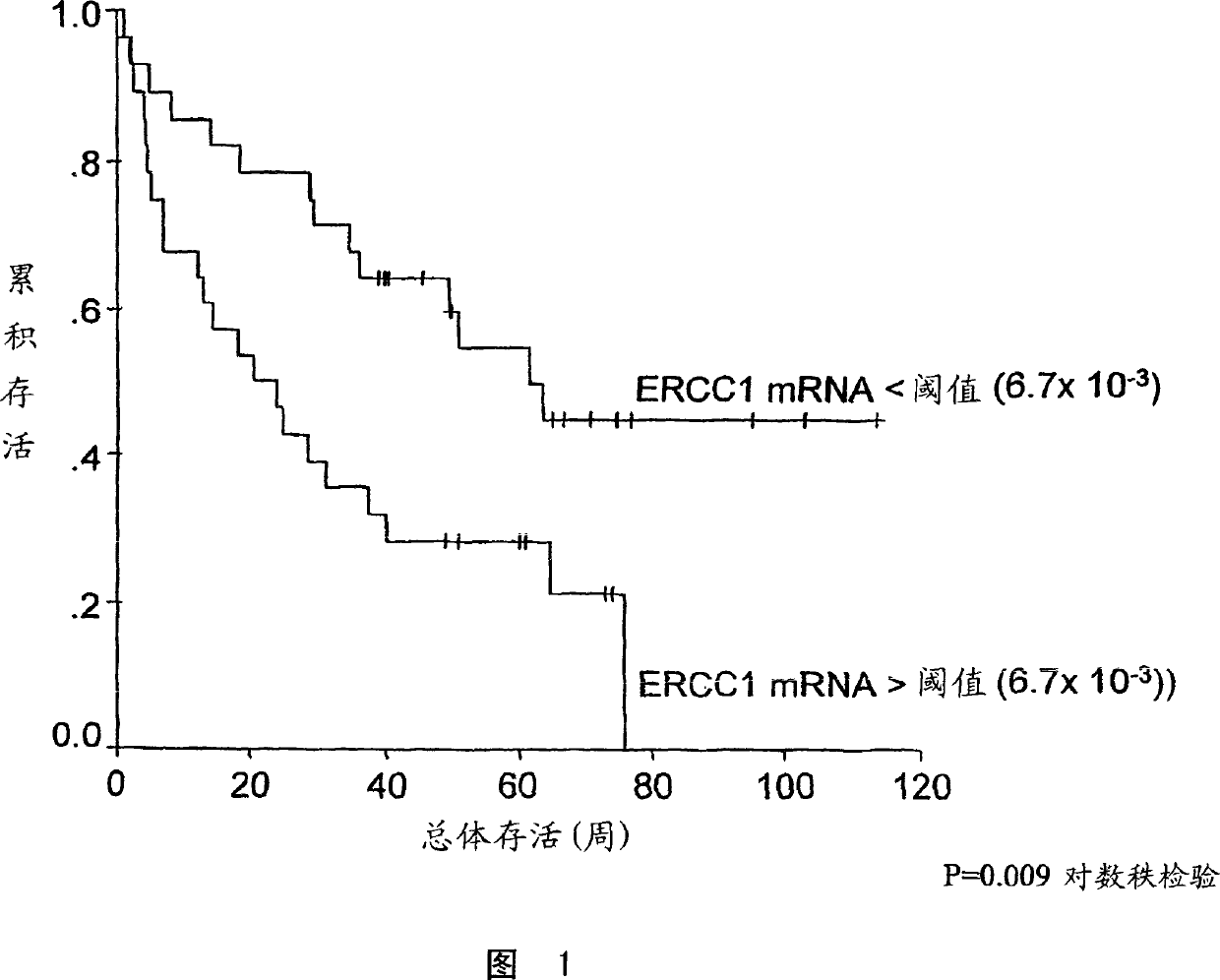
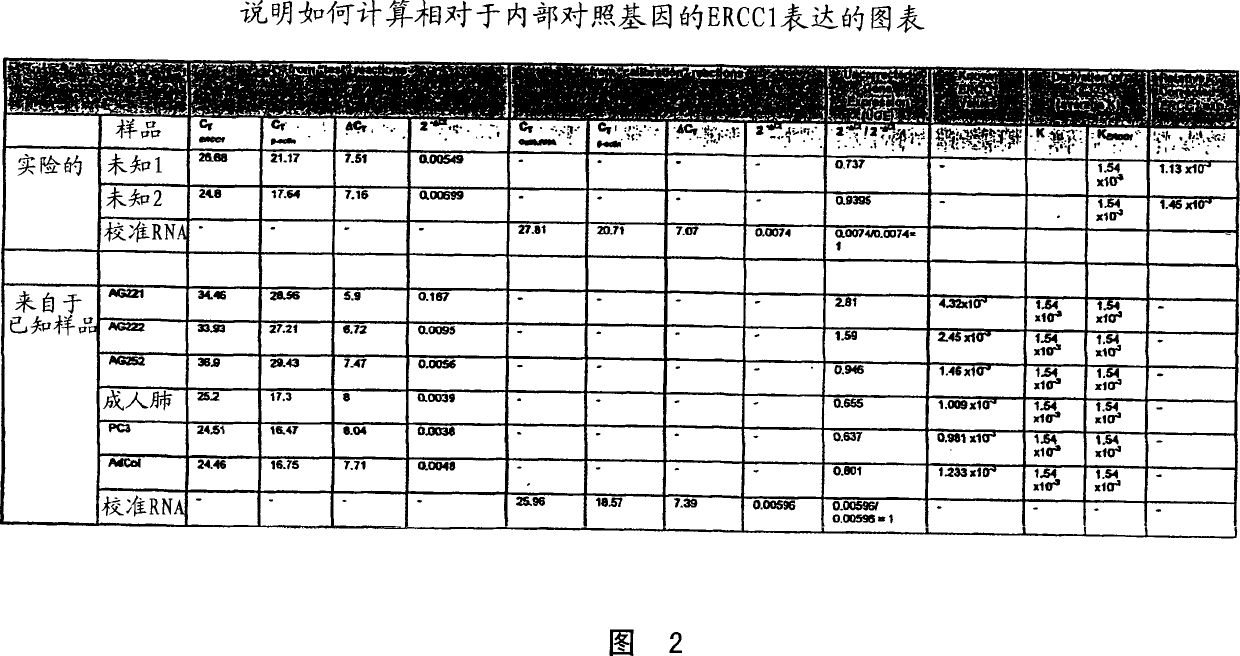
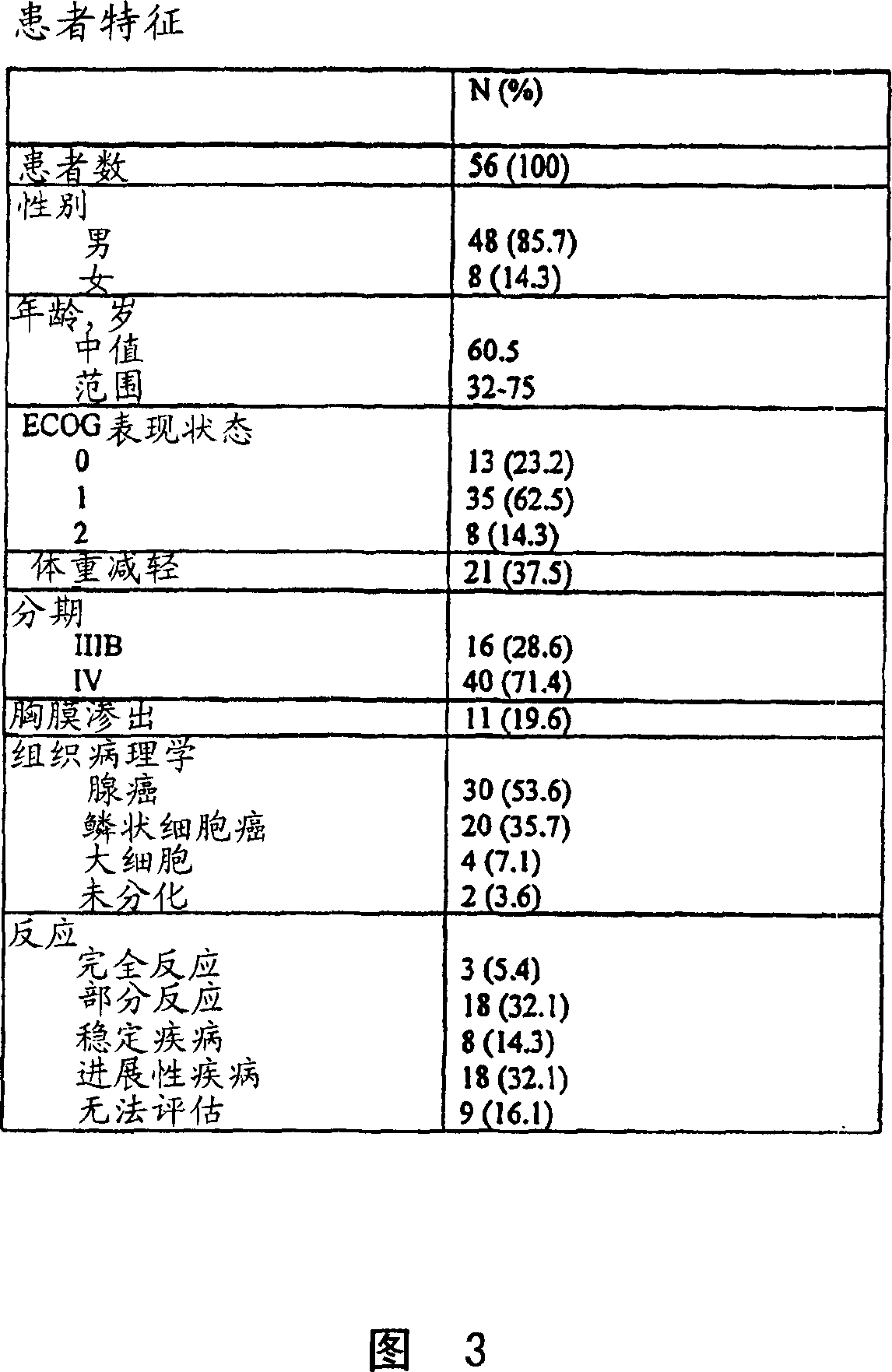
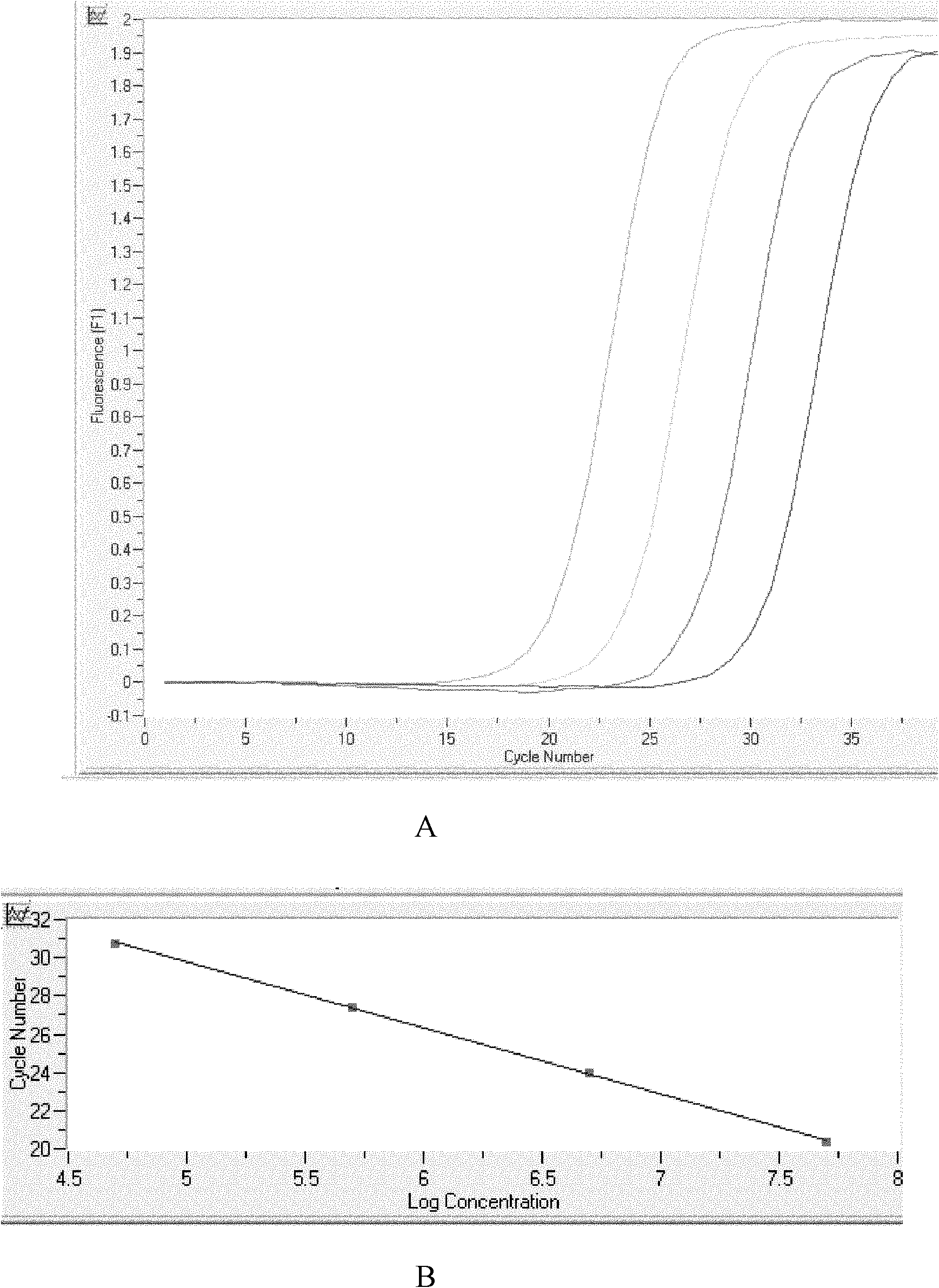
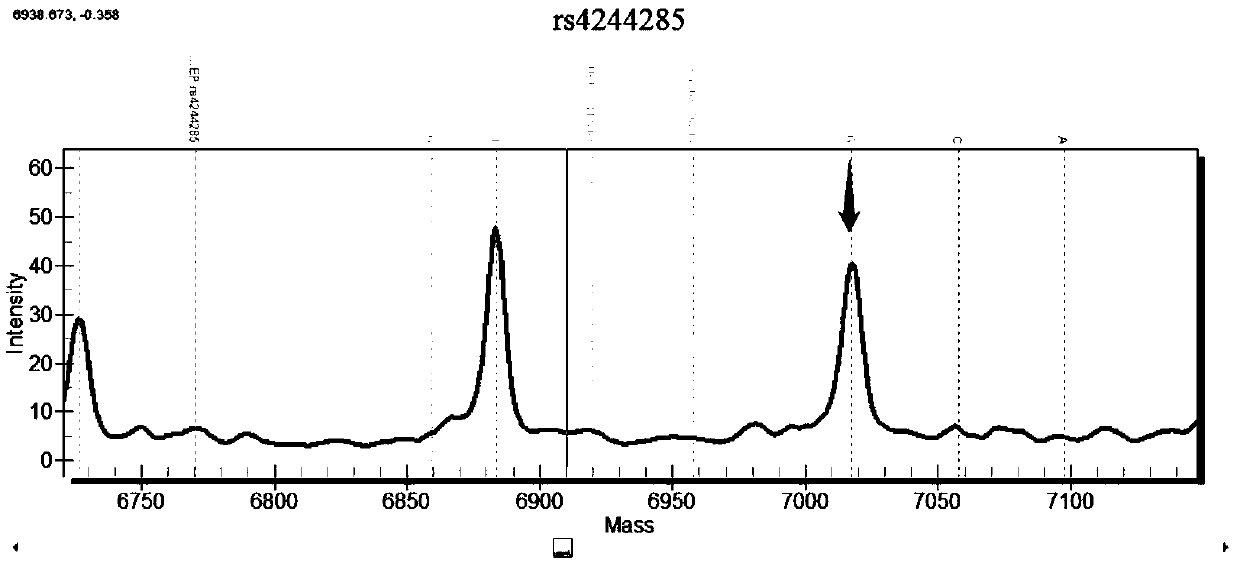
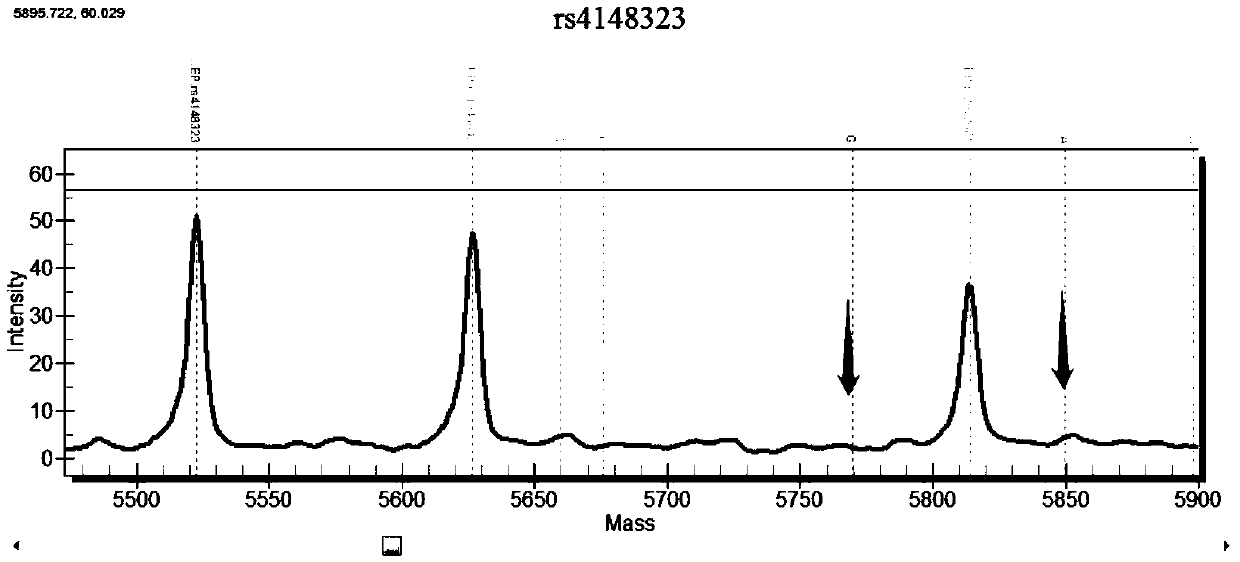
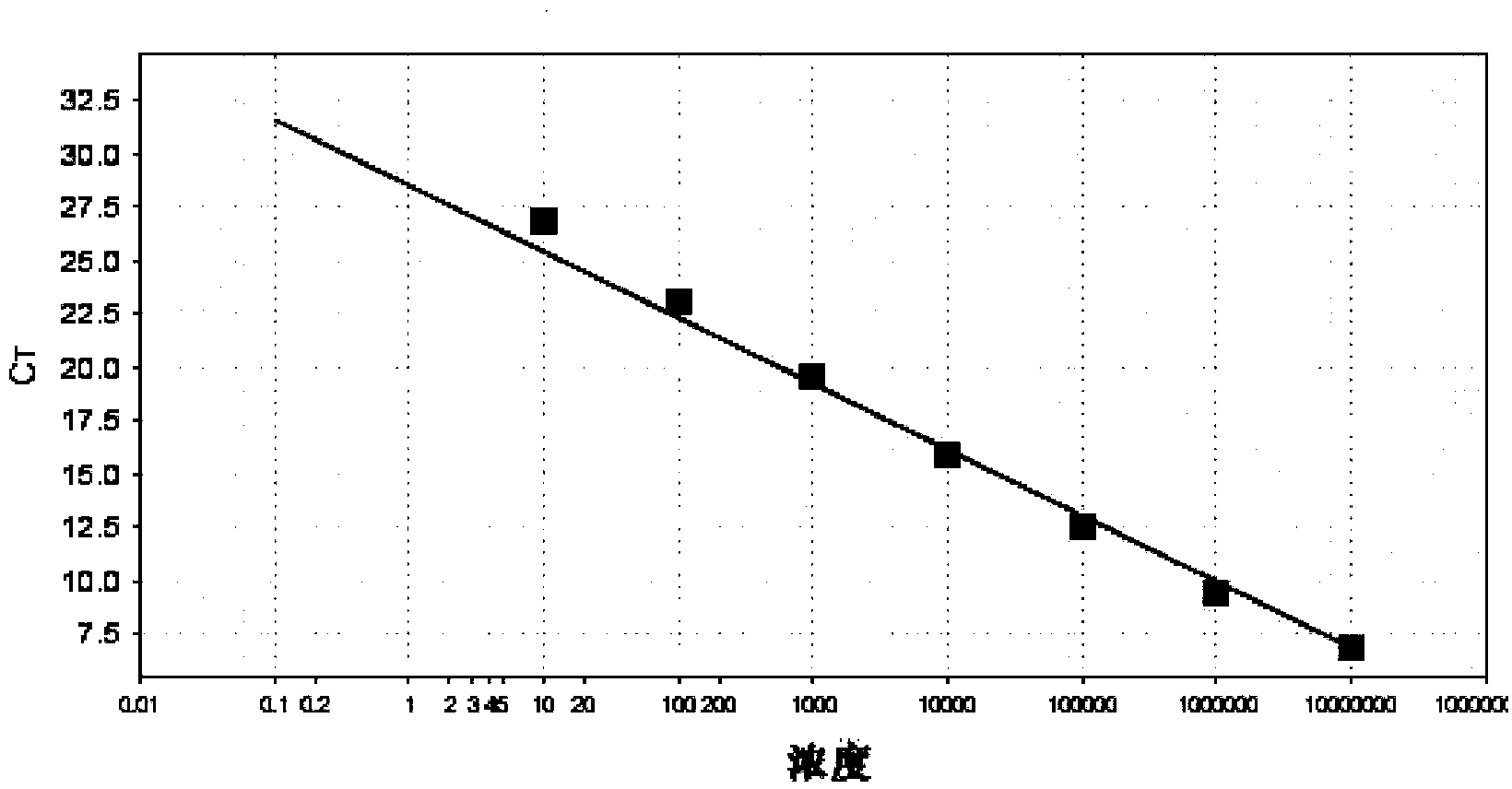
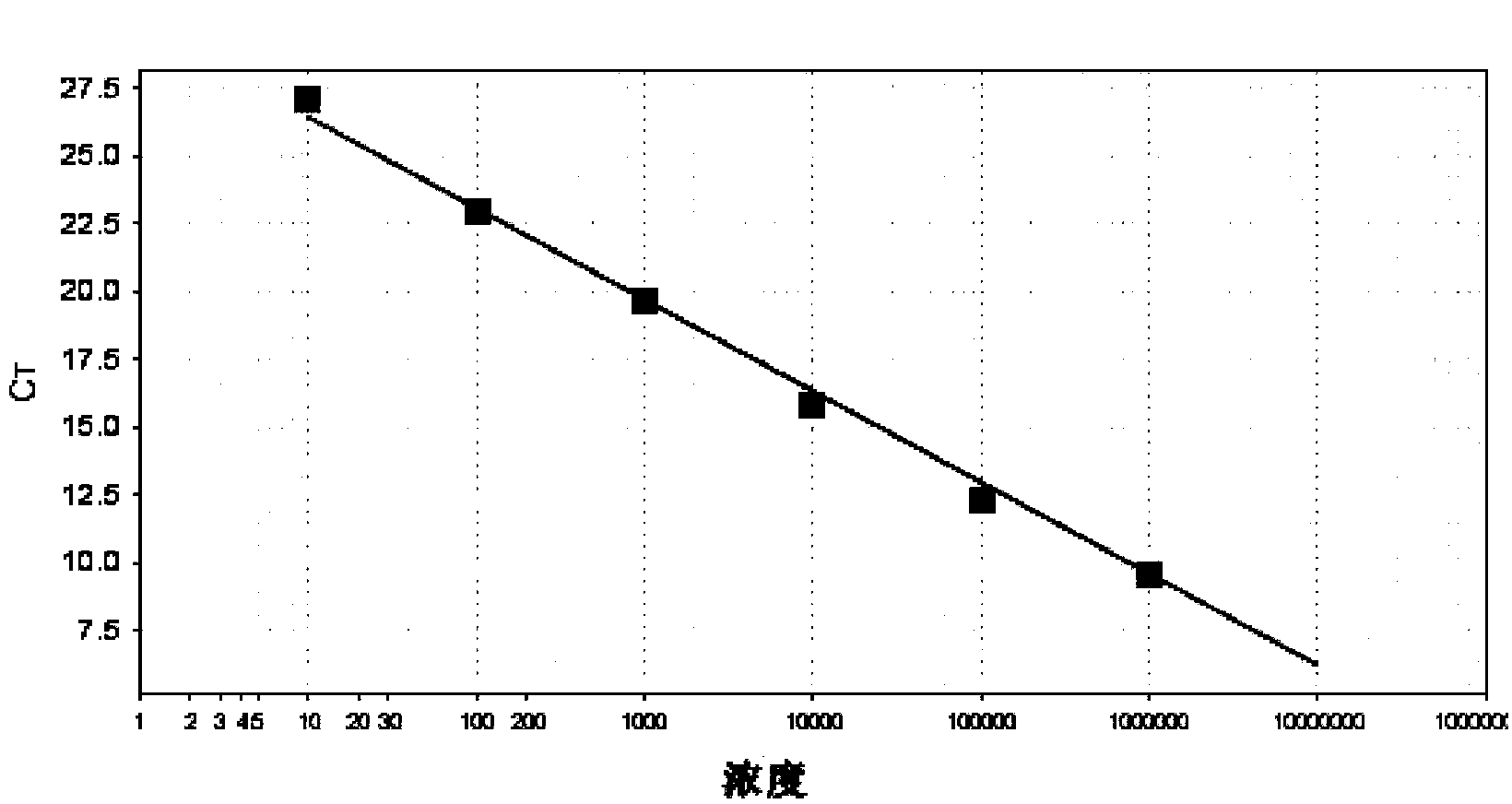
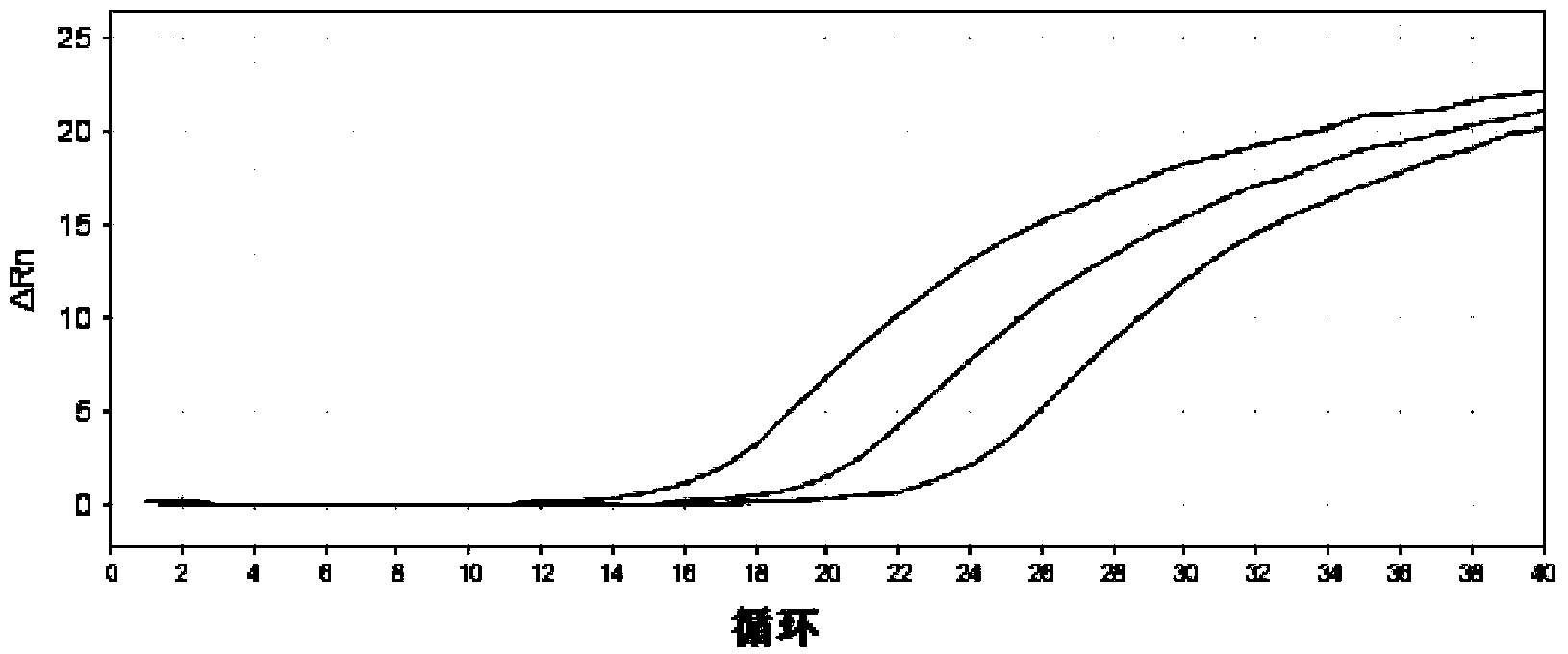
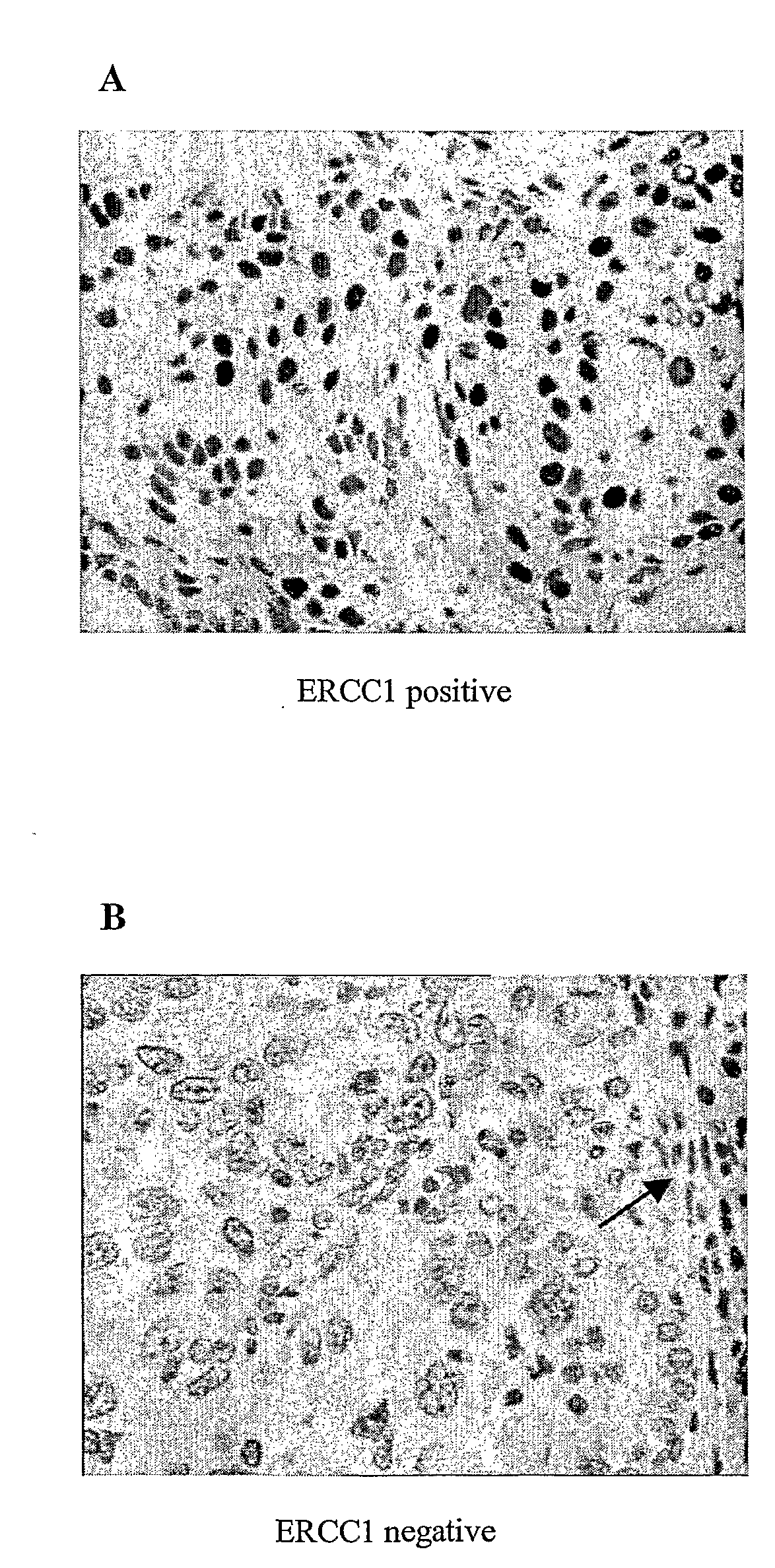
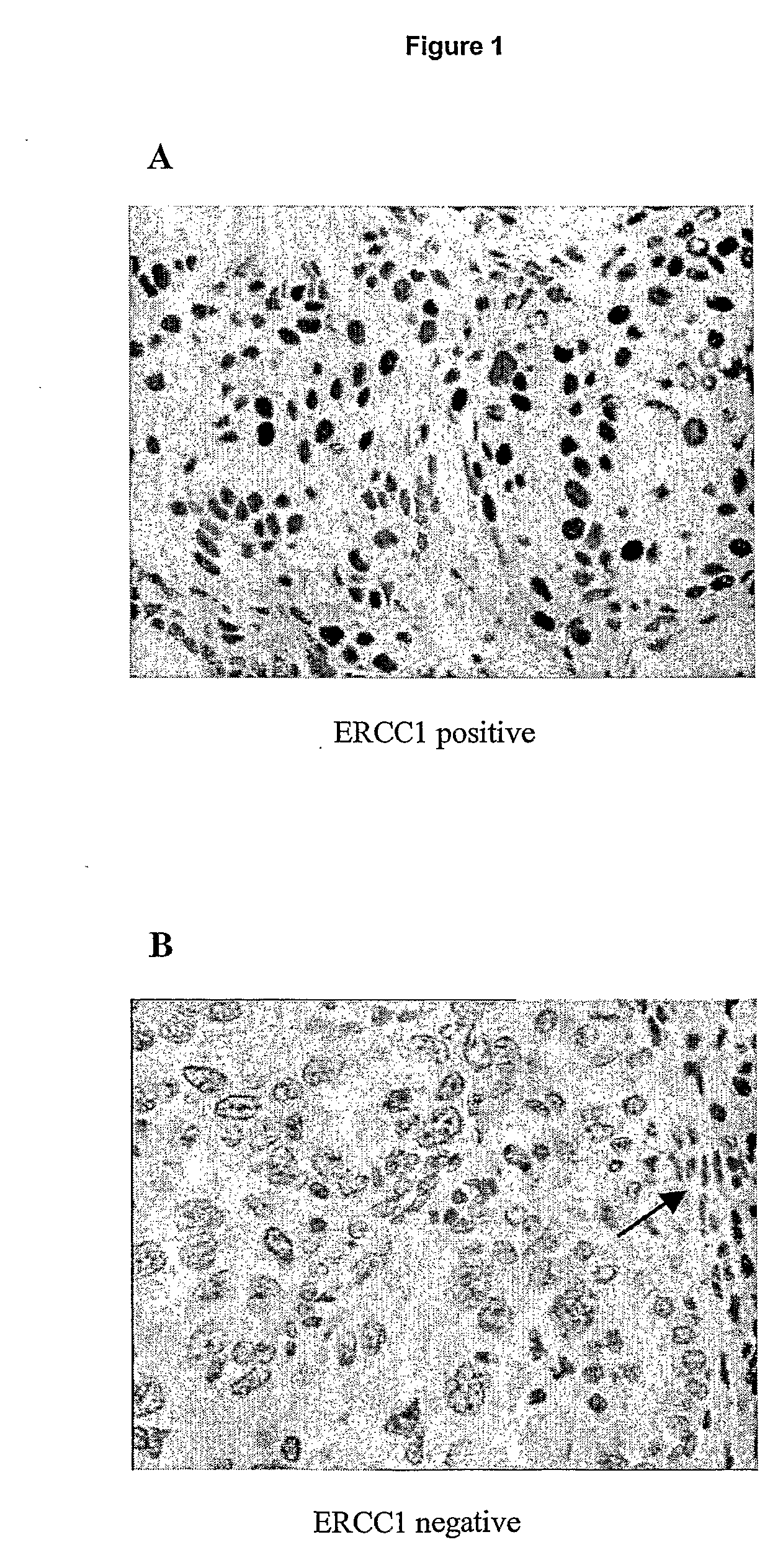
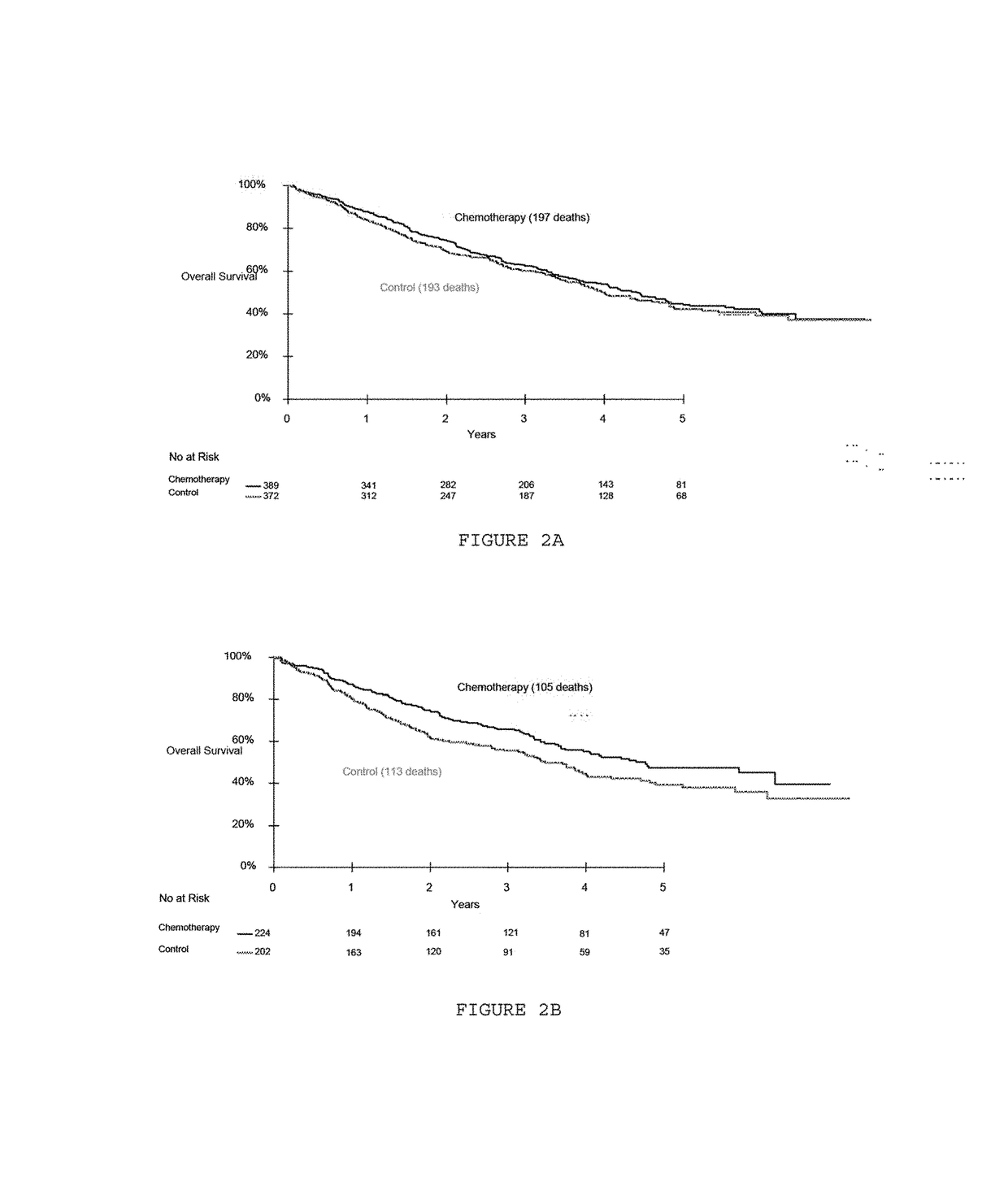
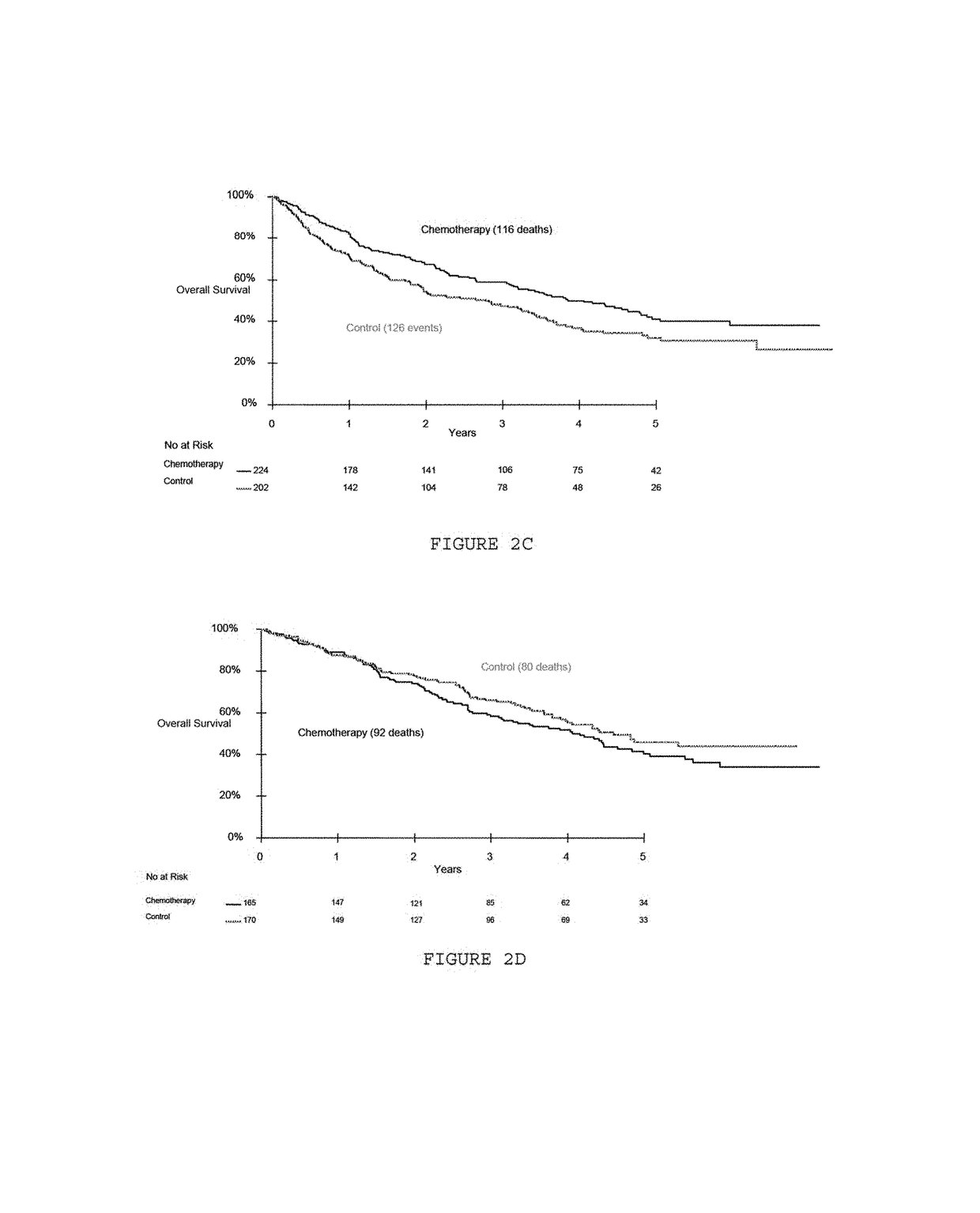
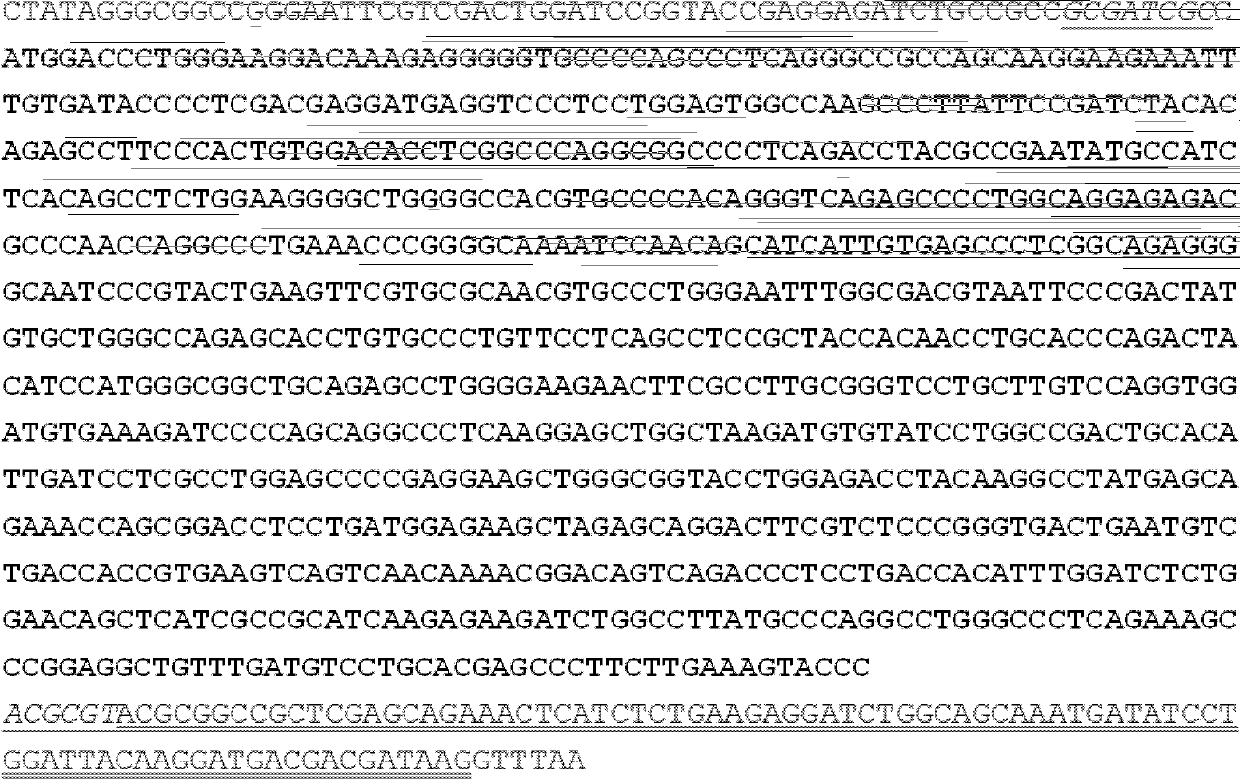
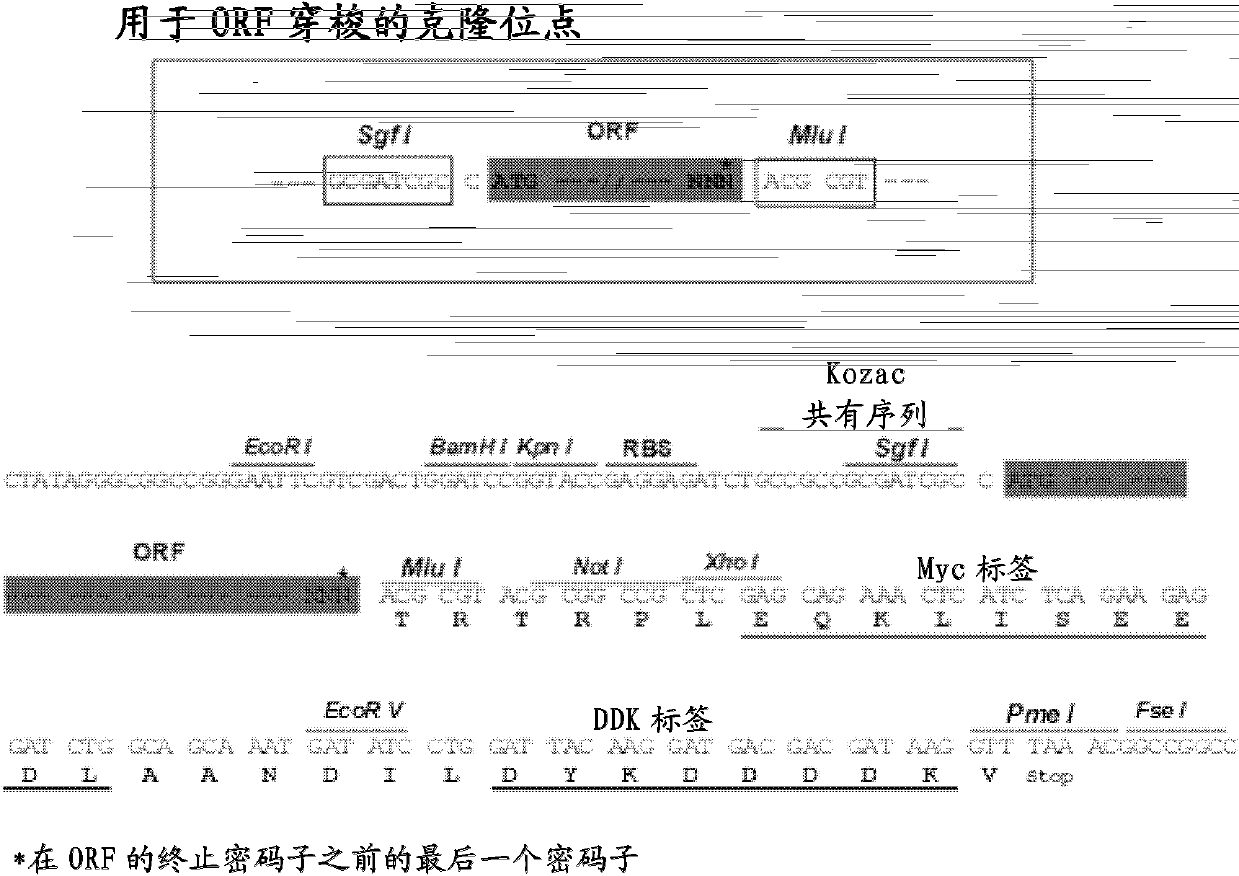
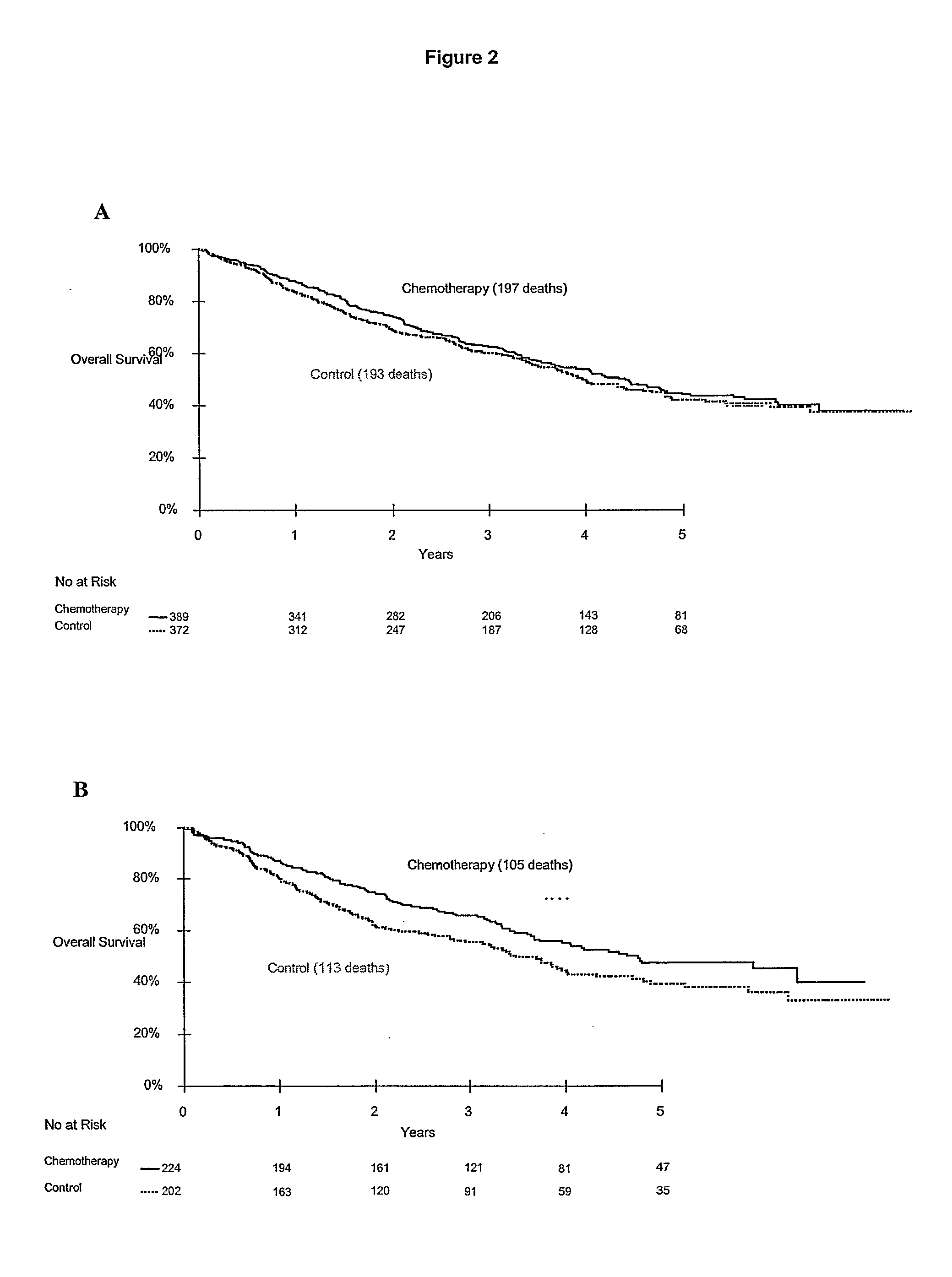Patents
Literature
47 results about "ERCC1" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
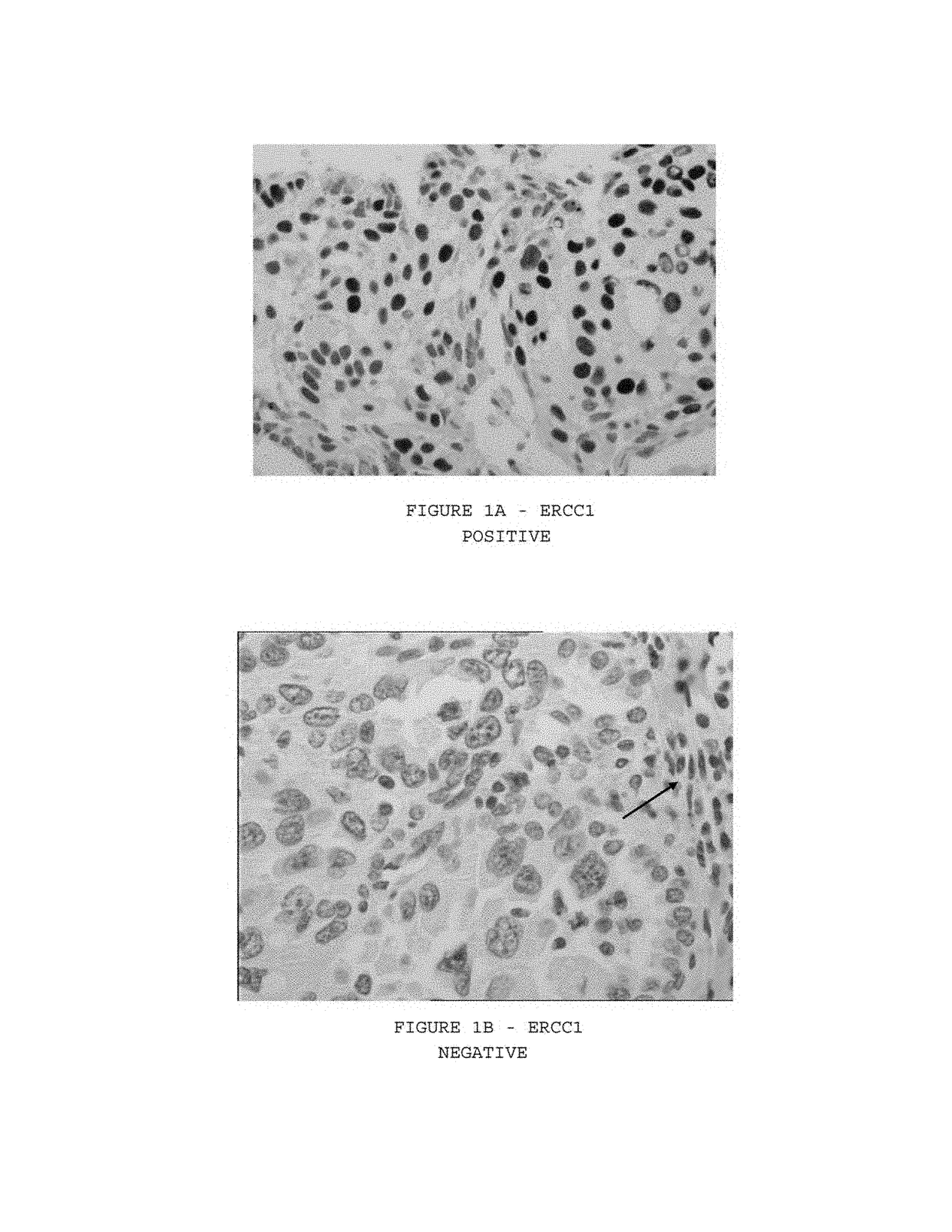
DNA excision repair protein ERCC-1 is a protein that in humans is encoded by the ERCC1 gene. Together with ERCC4, ERCC1 forms the ERCC1-XPF enzyme complex that participates in DNA repair and DNA recombination.
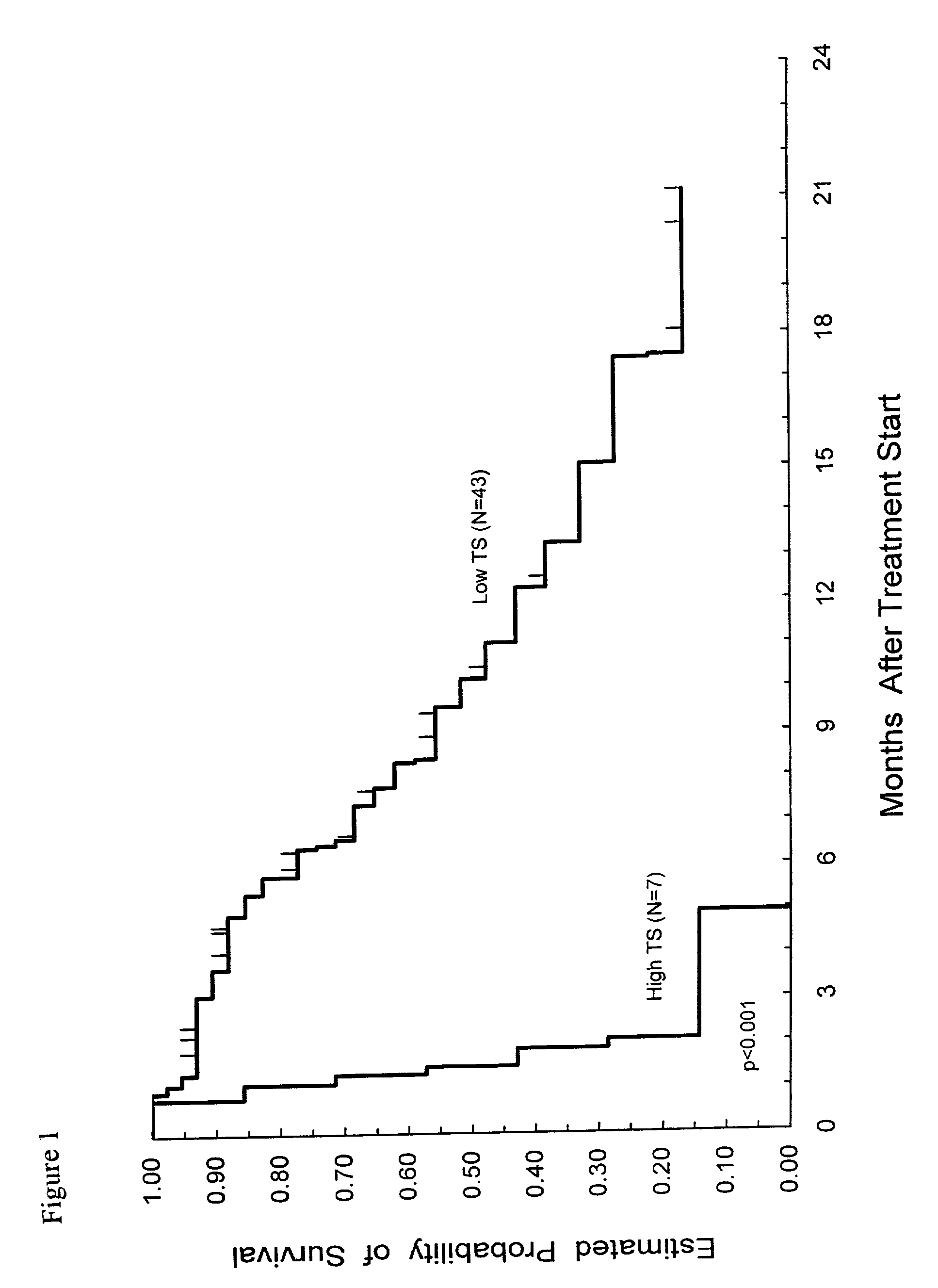
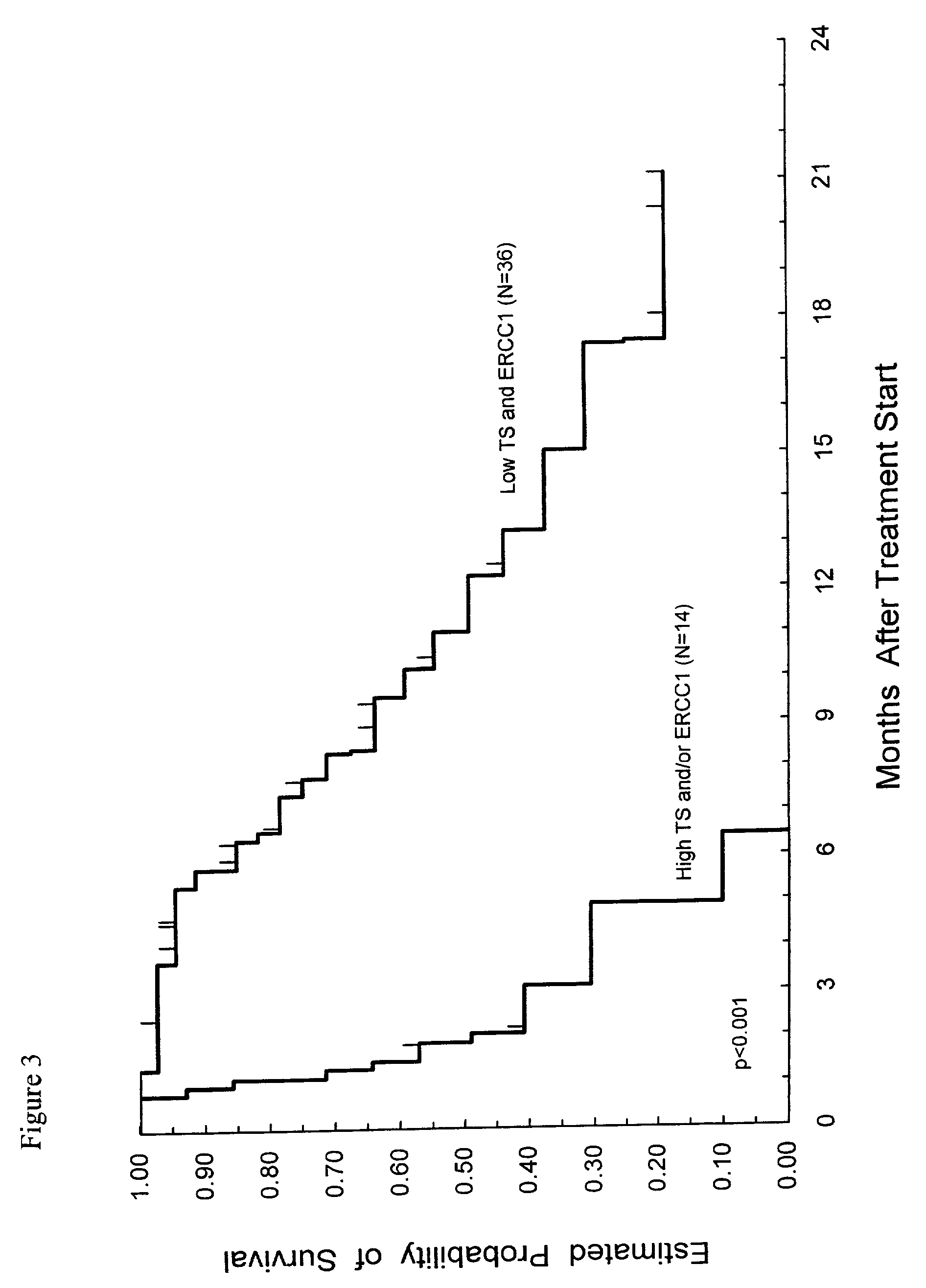
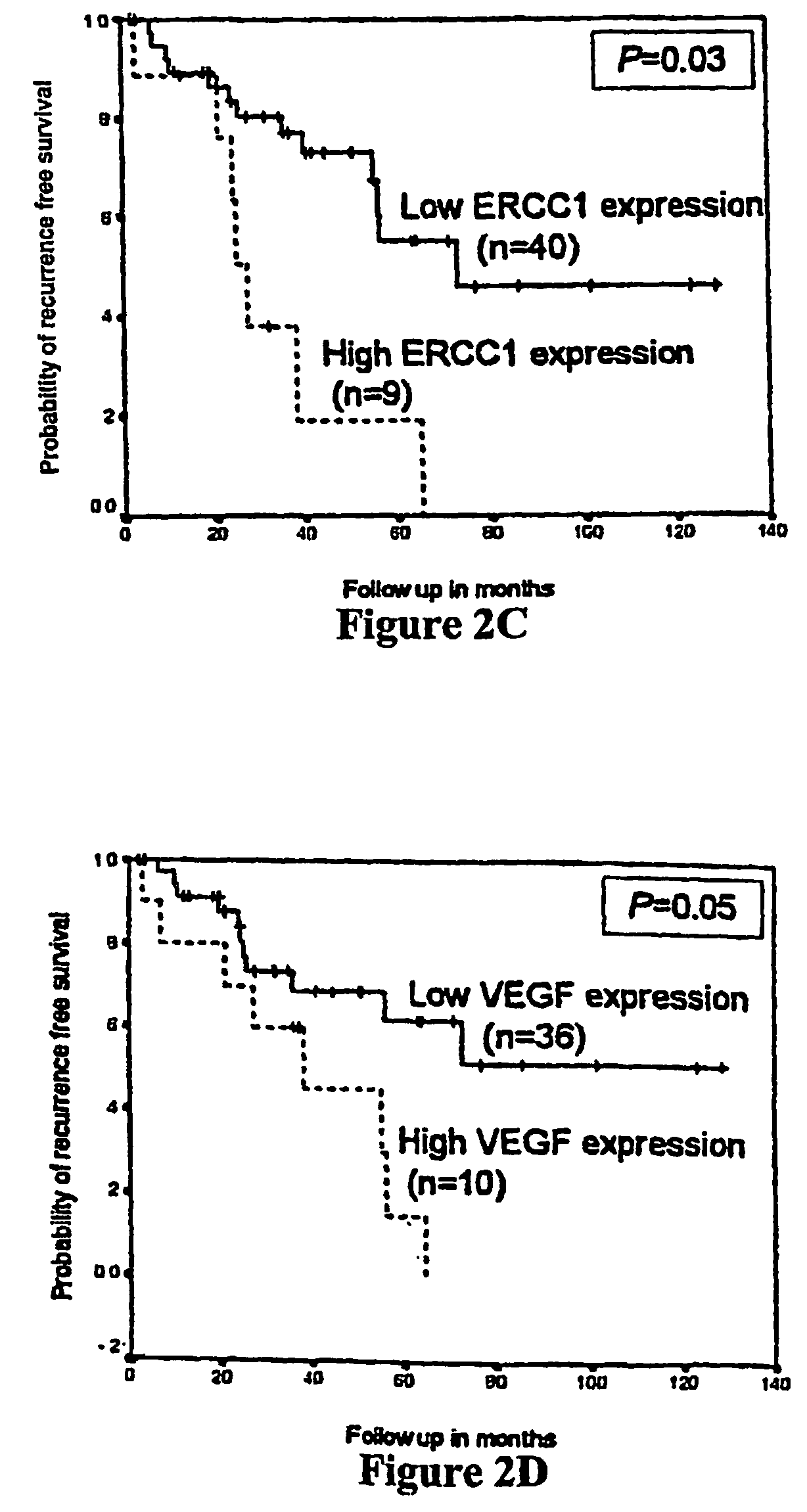
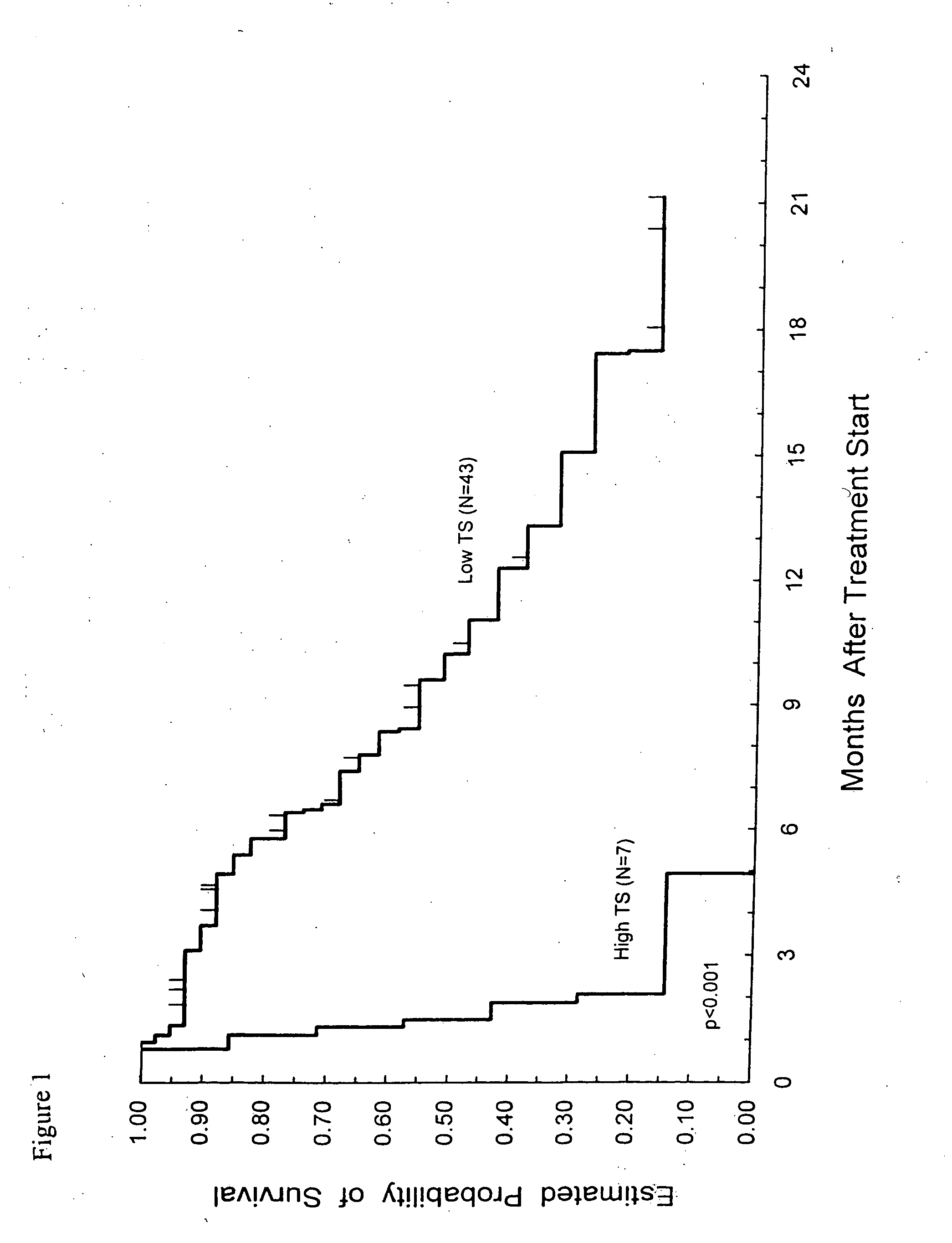
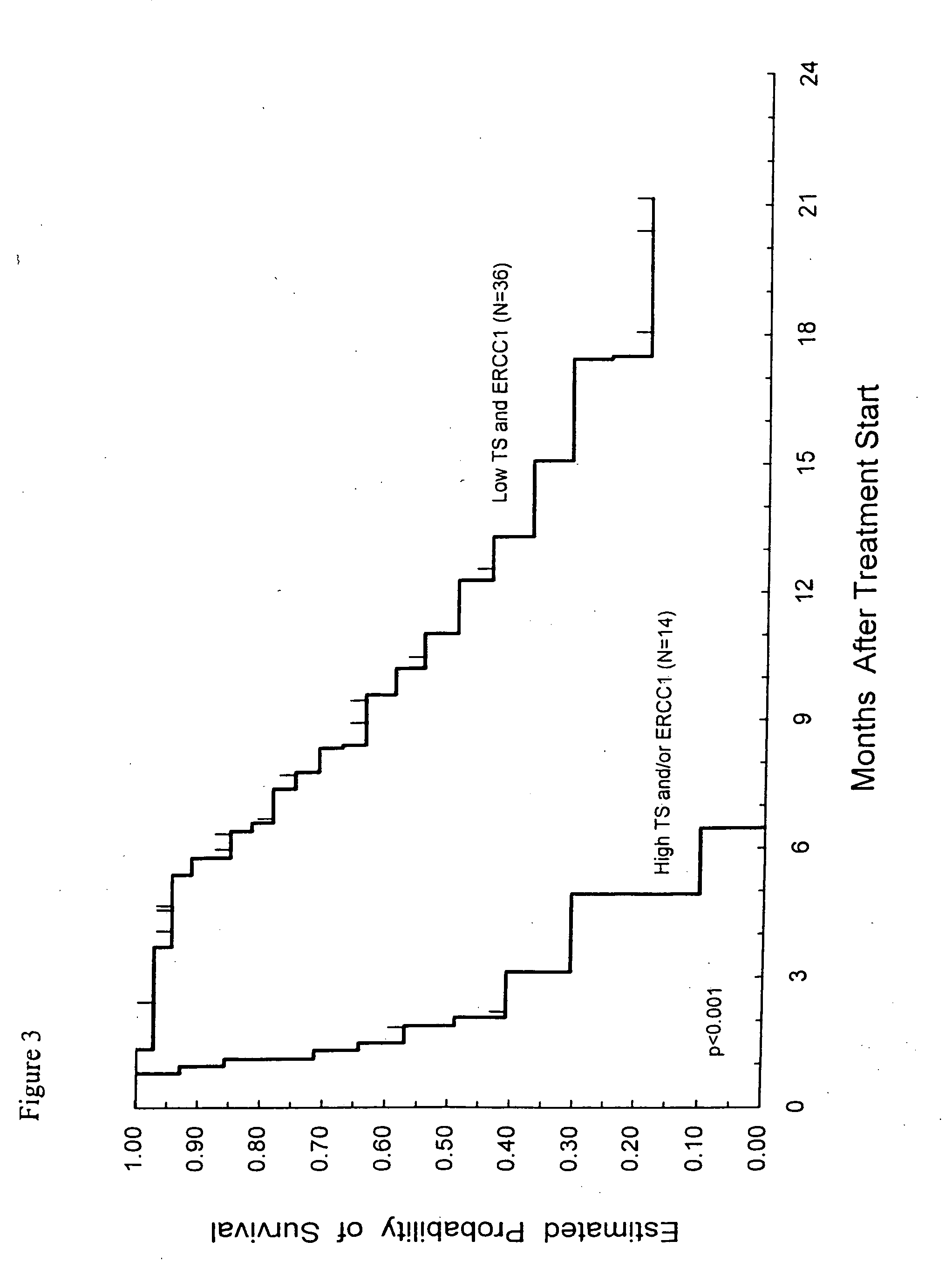
Method of determining a chemotherapeutic regimen based on ERCC1 and TS expression
InactiveUS7049059B2Improve responseMicrobiological testing/measurementRecombinant DNA-technologyAbnormal tissue growthRegimen
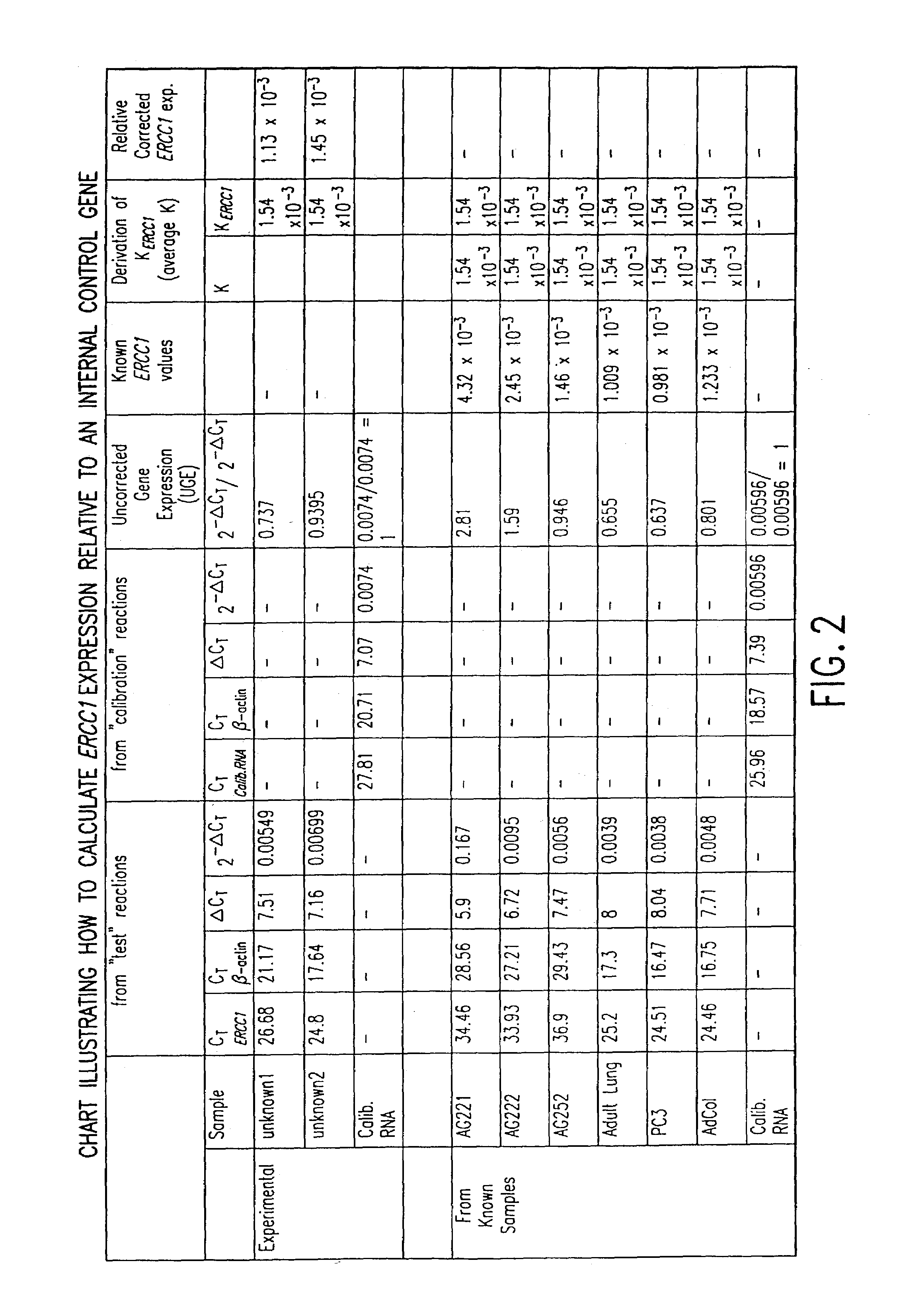
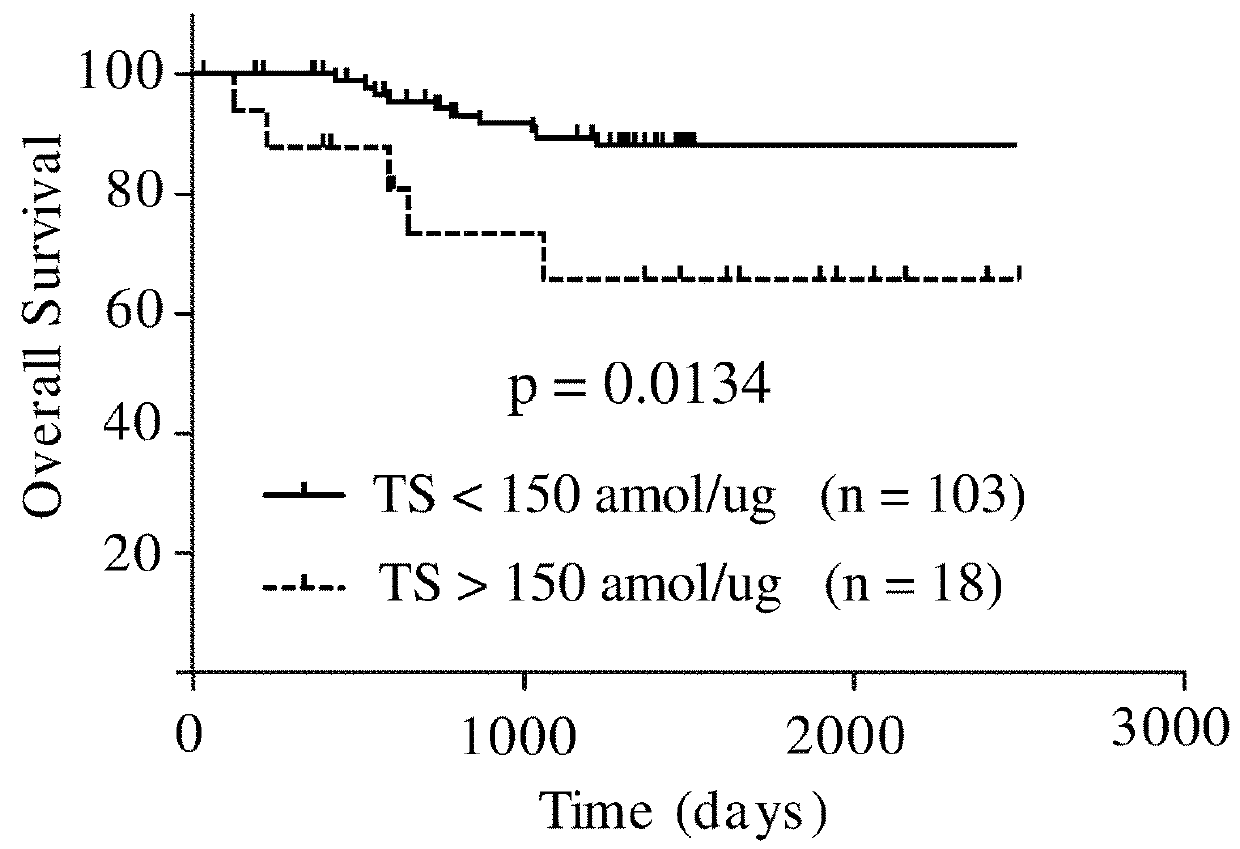
The present invention relates to prognostic methods which are useful in medicine, particularly cancer chemotherapy. The object of the invention to provide a method for assessing TS and / or ERCC1 expression levels in fixed or fixed and paraffin embedded tissues and prognosticate the probable resistance of a patient's tumor to treatment with 5-FU and oxaliplatin-based therapies by examination of the amount of TS and / or ERCC1 mRNA in a patient's tumor cells and comparing it to a predetermined threshold expression level for those genes. More specifically, the invention provides to oligonucleotide primer pairs ERCC1 and TS and methods comprising their use for detecting levels of ERCC1 and TS mRNA, respectively.
Owner:CANCER GENETICS
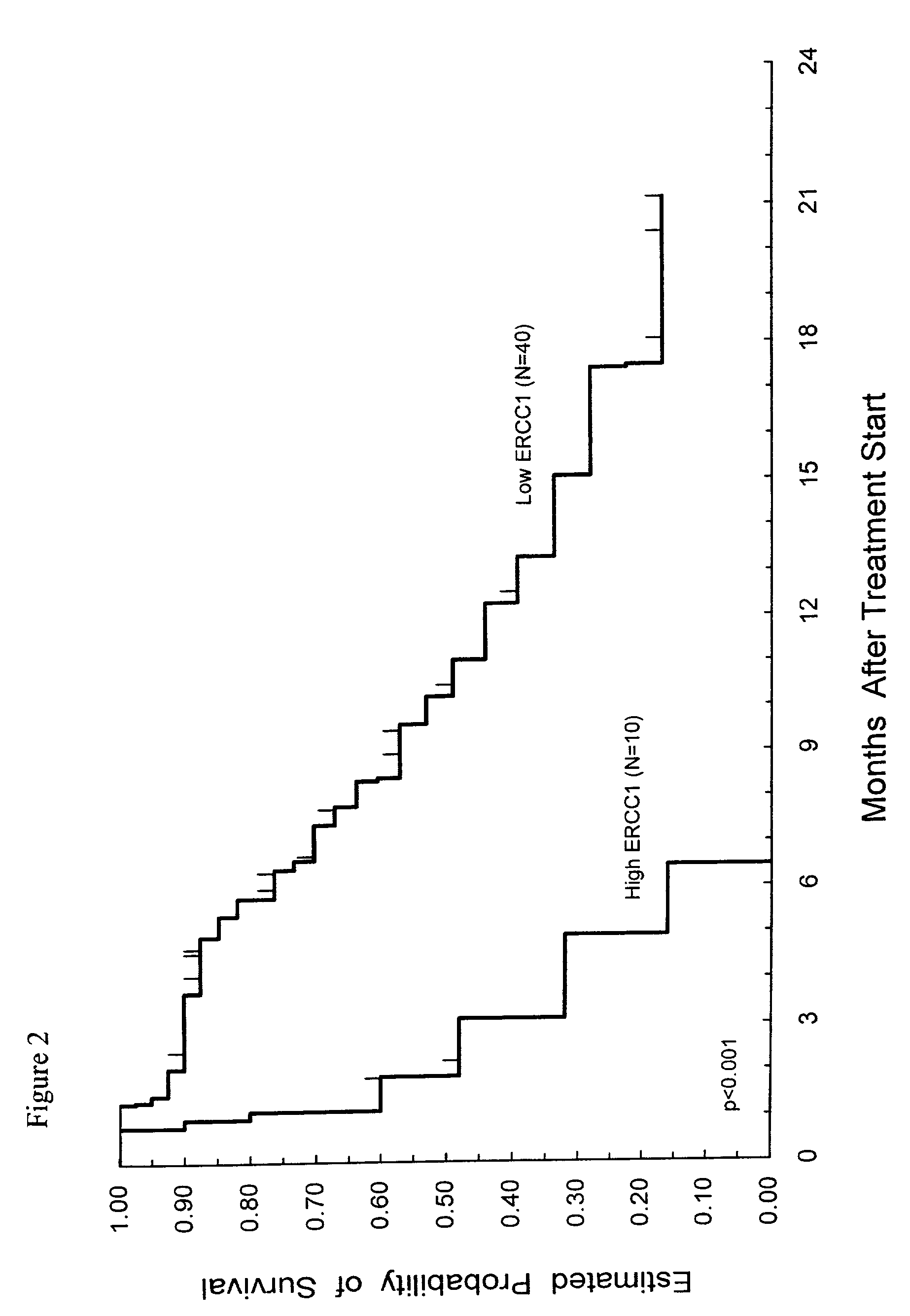
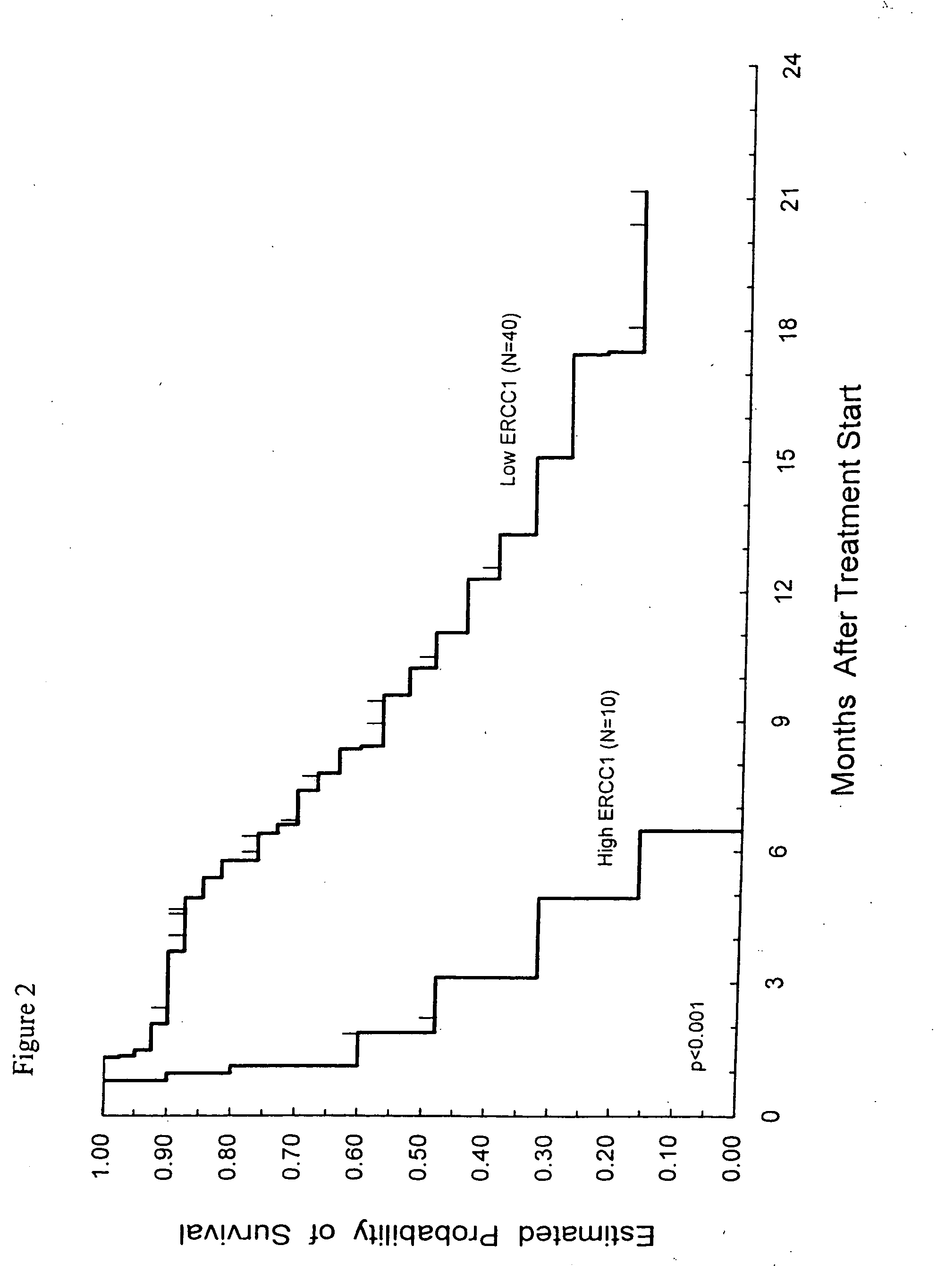
Method of determining a chemotherapeutic regimen based on ERCC1 expression
The present invention relates to prognostic methods which are useful in medicine, particularly cancer chemotherapy. The object of the invention to provide a method for assessing ERCC1 expression levels in fixed or fixed and paraffin embedded tissues and determine a platinum-based chemotherapy by examination of the amount of ERCC1 mRNA in a patient's tumor cells and comparing it to a predetermined threshold expression level. More specifically, the invention provides to oligonucleotide primer pair ERCC1 and methods comprising their use for detecting levels of ERCC1 mRNA.
Owner:CANCER GENETICS
Polymorphisms in the ERCC1 gene for predicting treatment outcome
The invention provides compositions and methods for determining the increased risks for recurrence of certain cancers and the likelihood of successful treatment with one or both of chemotherapy and radiation therapy. The methods comprising determining the type of genomic polymorphism present in a predetermined region of the gene of interest isolated from the subject or patient. Also provided are nucleic acid probes and kits for determining a patient's cancer risk and treatment response.
Owner:UNIV OF SOUTHERN CALIFORNIA
Method of determining a chemotherapeutic regimen based on ERCC1 and TS expression
InactiveUS20060121526A1Sugar derivativesMicrobiological testing/measurementAbnormal tissue growthRegimen
The present invention relates to prognostic methods which are useful in medicine, particularly cancer chemotherapy. The object of the invention to provide a method for assessing TS and / or ERCC1 expression levels in fixed or fixed and paraffin embedded tissues and prognosticate the probable resistance of a patient's tumor to treatment with 5-FU and oxaliplatin-based therapies by examination of the amount of TS and / or ERCC1 mRNA in a patient's tumor cells and comparing it to a predetermined threshold expression level for those genes. More specifically, the invention provides to oligonucleotide primer pairs ERCC1 and TS and methods comprising their use for detecting levels of ERCC1 and TS mRNA, respectively.
Owner:CANCER GENETICS INC
Method of determining a chemotherapeutic regimen based on ERCCI expression
The present invention relates to predictive methods for use in medicine, in particular cancer chemotherapy. The purpose of the present invention is to provide a method for evaluating the expression level of ERCC1 in fixed or fixed and paraffin-embedded tissues by detecting the amount of ERCC1 mRNA in tumor cells of a patient and comparing it with a predetermined threshold expression level, and determining the platinum-based Methods of chemotherapy. More particularly, the present invention provides oligonucleotide primer pairs for ERCC1, and methods for detecting ERCC1 mRNA levels using them.
Owner:RESPONSE GENETICS
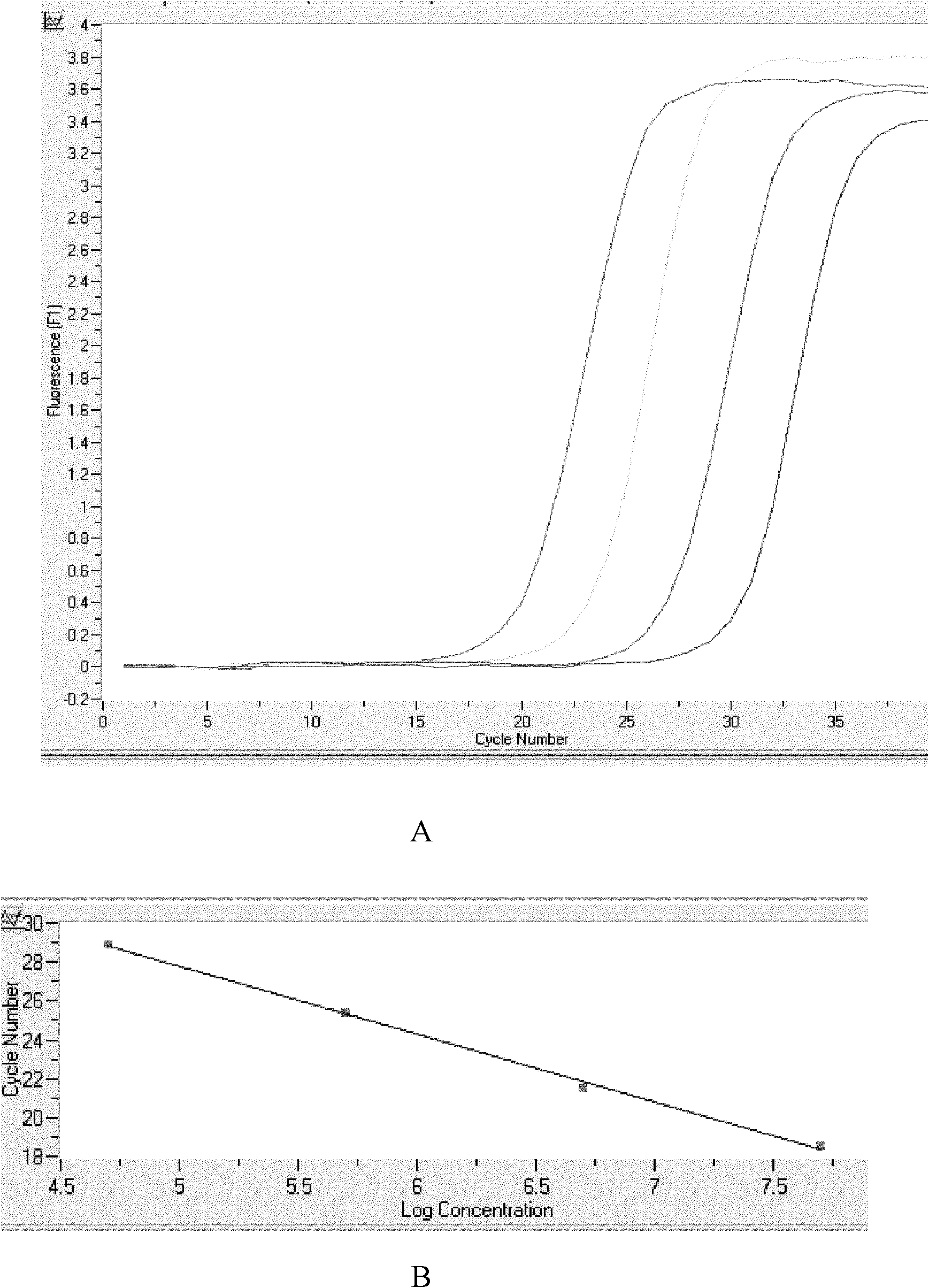
Kit for detecting ERCC1 mRNA (Excision Repair Cross Complement Group 1 Messenger Ribonucleic Acid) expression by using fluorescence quantitative PCR (Polymerase Chain Reaction) technology
InactiveCN102154475AHigh detection sensitivityImprove accuracyMicrobiological testing/measurementFluorescence/phosphorescenceExcision repair cross-complementingFluorescence
The invention relates to the field of biotechnology, discloses a fluorescence quantitative PCR (Polymerase Chain Reaction) diagnostic kit for rapidly detecting ERCC1 mRNA (Excision Repair Cross Complement Group 1 Messenger Ribonucleic Acid), and aims to provide a kit capable of rapidly, conveniently, sensitively and specifically detecting ERCC1 mRNA and application thereof. An expression status of a DNA (Deoxyribonucleic Acid) repair gene can be used as a relatively ideal predictor for a chemotherapeutic effect. Nucleotide excision repair (NER) is in close relation with platinum drug resistance, wherein the excision repair cross complement group 1 (ERCC1) is an important factor which plays a role in a repair process and causes platinum drug resistance; the ERCC1 mRNA level is detected by using higher-sensitivity and higher-specificity fluorescence quantitative PCR; and the specificity and the sensitivity of a detection result are obviously improved. The kit provides a completely new rapid and convenient gene diagnosis technology for use of platinum type chemotherapeutic medicaments of clinical malignant tumor patients.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
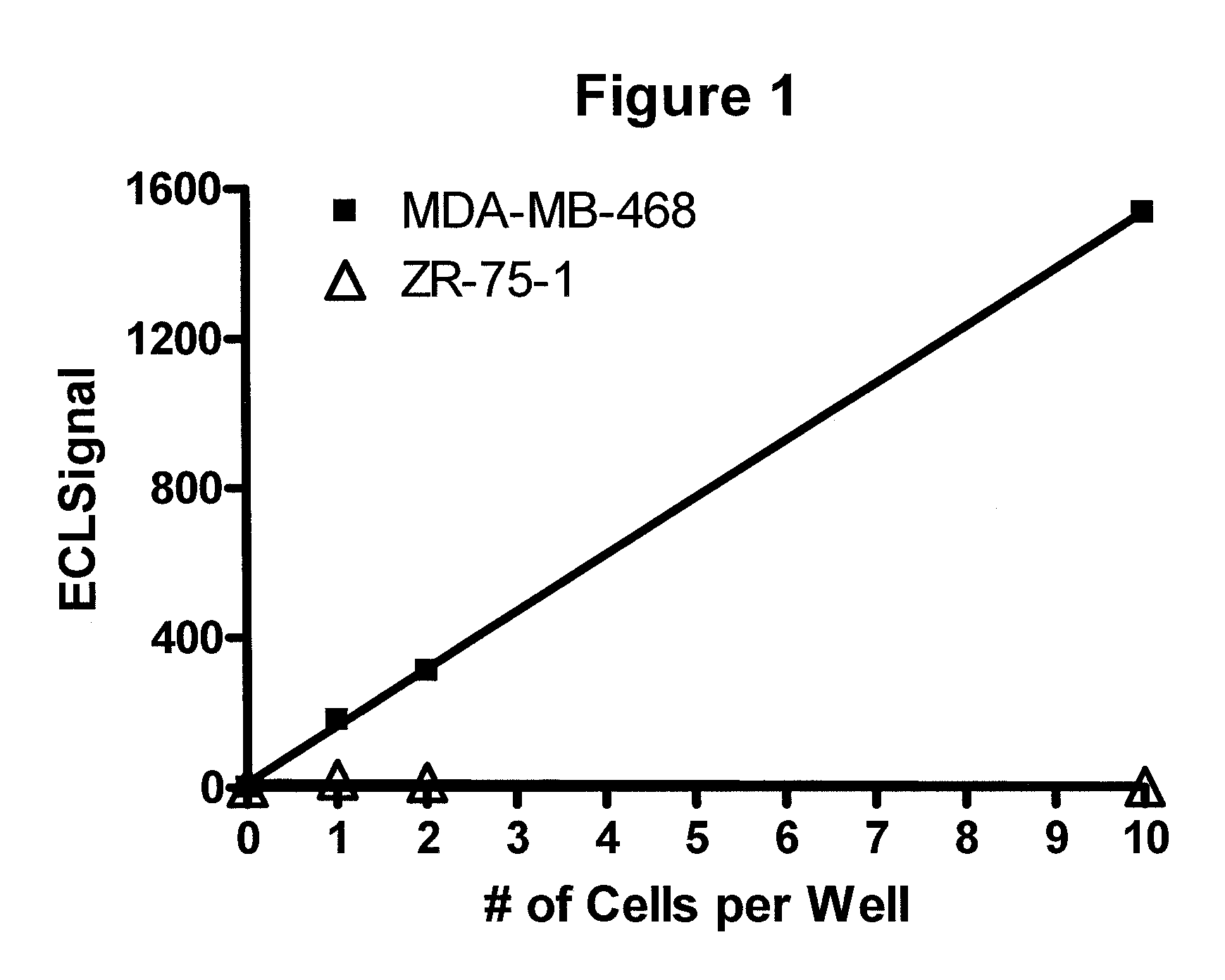
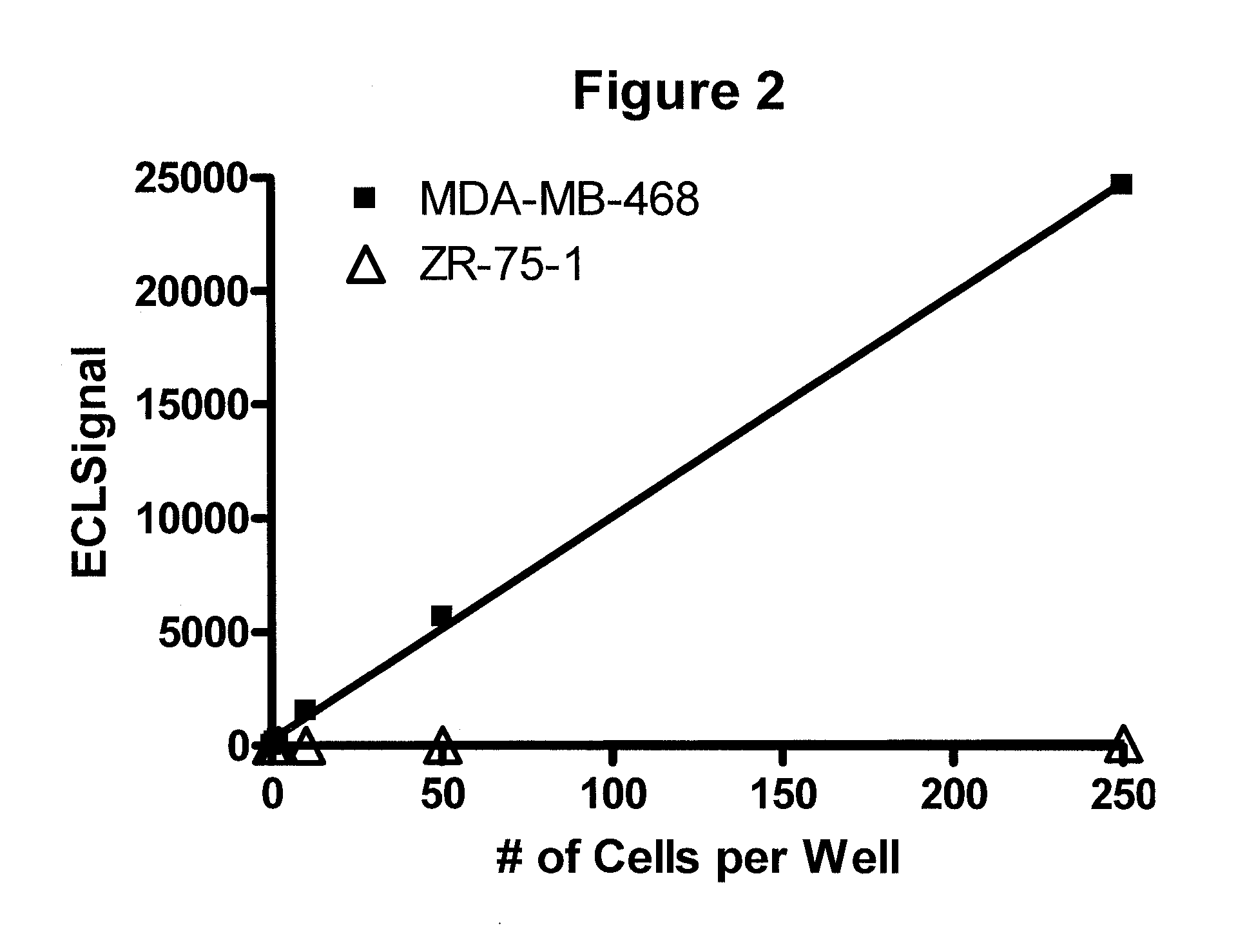
Detection of proteins from circulating neoplastic cells
InactiveUS20090098138A1The process is convenient and fastPeptide/protein ingredientsAntibody ingredientsErlotinibCancer cell
The protein EGFR, ERCC1, RRM1, thymidylate synthase, or beta-tubulin from cancer cells is detected in a blood sample by enriching the cancer cells from the blood sample followed by performing on the enriched cancer cells an immunoassay capable of detecting the proteins mentioned above. Cancer patients overexpressing EGFR are treated with an anti-EGFR agent, for example cetuximab, panitumumab, erlotinib or gefitinib.
Owner:WELLSTAT BIOLOGICS CORP
Multiple-gene detecting kit related to antitumor drugs
ActiveCN103882138AEasy to analyzeReliable analysisMicrobiological testing/measurementDPYDReference genes
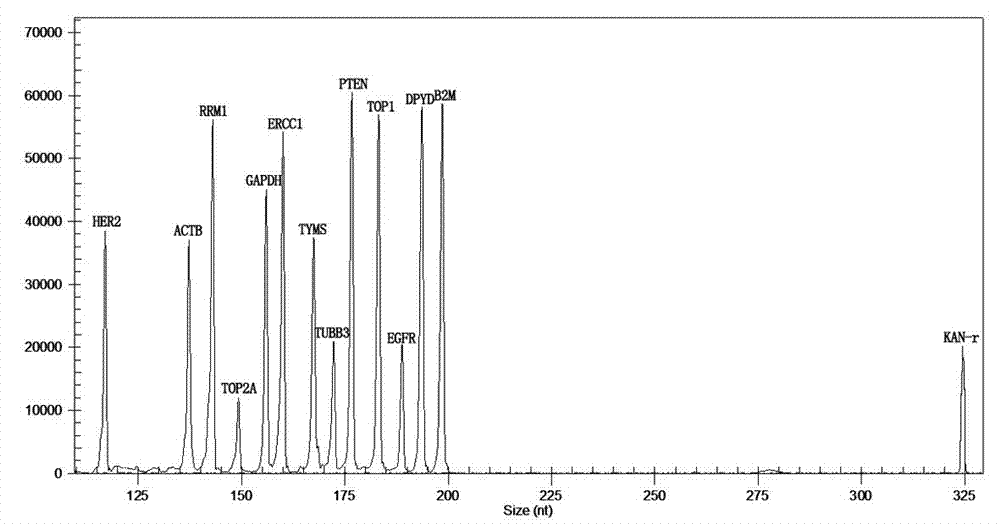
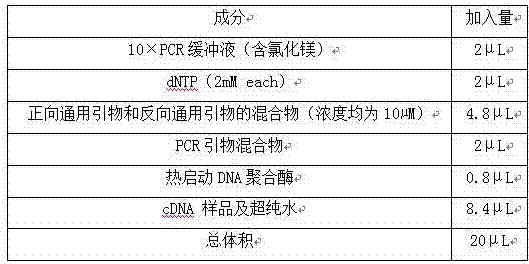
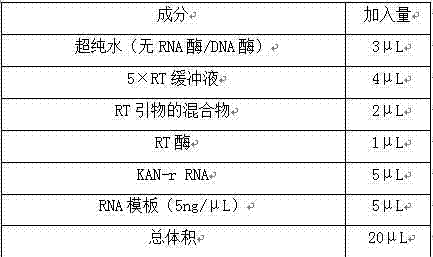
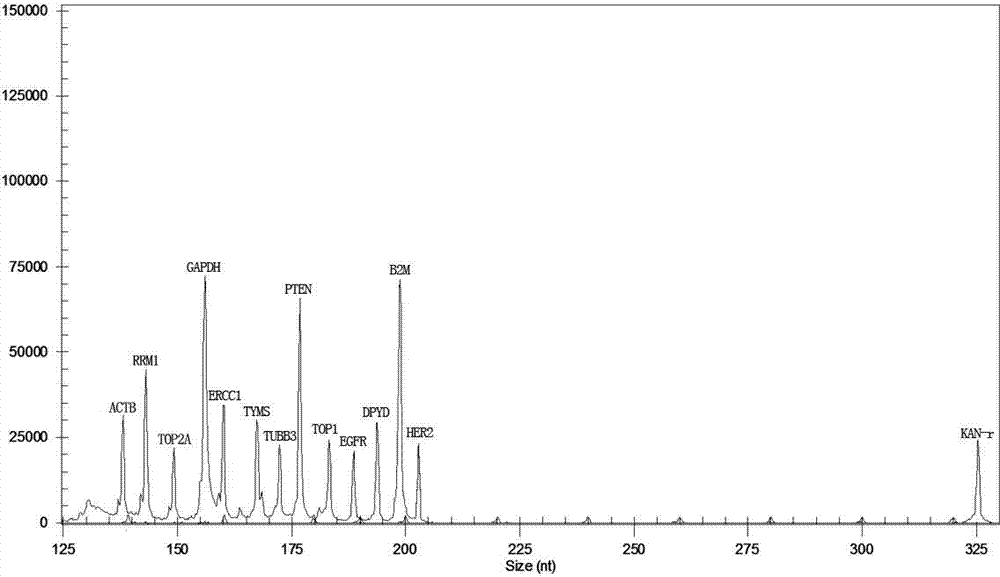
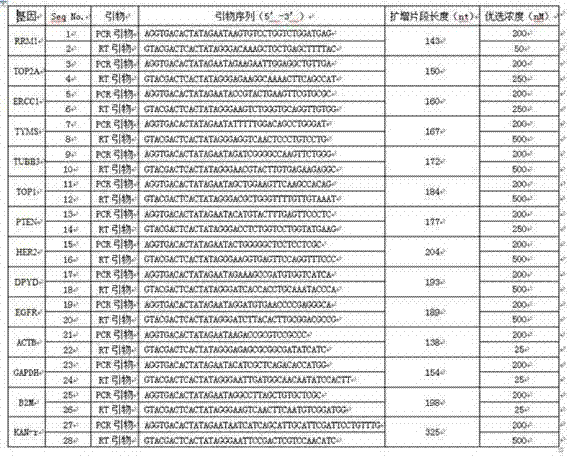
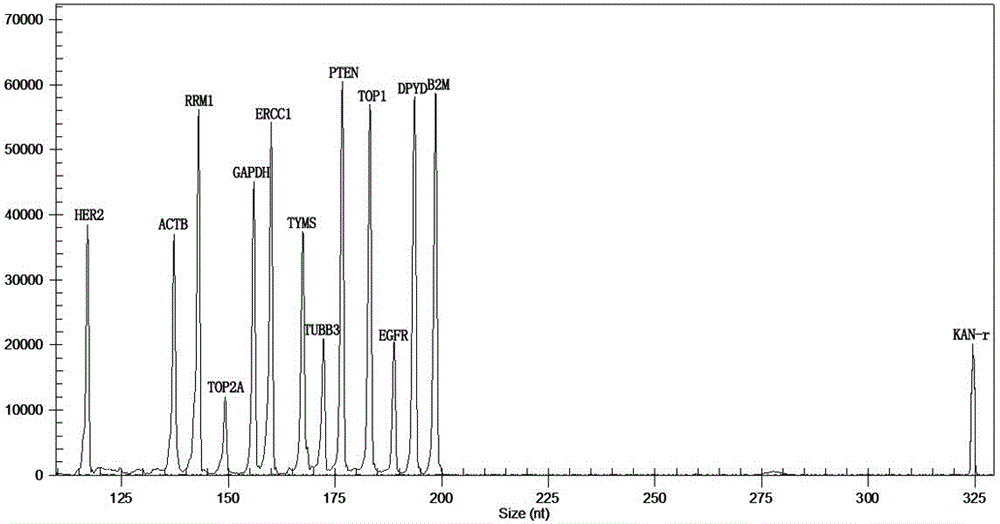
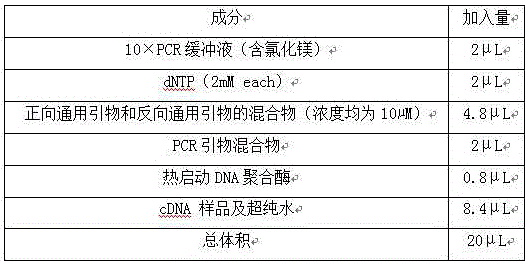
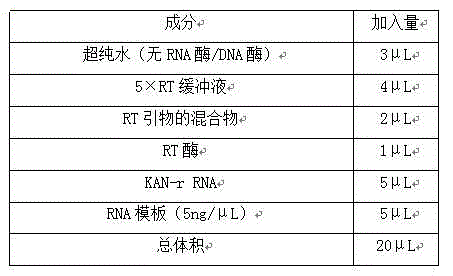
The invention relates to a multiple-gene detecting kit related to antitumor drugs. The multiple-gene detecting kit comprises a mixture of RT (reverse transcription) primers and a mixture of PCR (polymerase chain reaction) amplification primers, wherein each RT primer and each PCR amplification primer based on genetic groups, reference genes and a reaction internal label are respectively contained in each of the mixture of the RT primers and the mixture of the PCR amplification primers; the multiple-gene detecting kit is characterized in that the genetic groups comprise PTEN, EGFR, DPYD, HER2, RRM1, ERCC1, TUBB3, TOP1, TYMS and TOP2A; the reference genes comprise ACTB, GAPDH and B2M; the reaction internal label is KAN-rRNA (ribosomal RNA). The multiple-gene detecting kit related to antitumor drugs disclosed by the invention can be used for systemically detecting the expression level of a plurality of genes closely related to the antitumor drugs by one step, is simple and convenient to use, good in accuracy and high in detecting efficiency, and can be used for guiding chemotherapy drugs and selecting chemotherapy regimens very well.
Owner:南昌市赛尔医药科技有限公司
Srm assays to chemotherapy targets
InactiveUS20170168057A1Easy to carryControl oxidationMass spectrometric analysisDisease diagnosisERCC1Mass Spectrometry-Mass Spectrometry
Methods are provided for quantifying the ENT1, ERCC1, FOLR1, RRM1, TUBB3, TOPO1, and / or TOPO2A proteins directly in biological samples that have been fixed in formalin by the method of Selected Reaction Monitoring (SRM) / Multiple Reaction Monitoring (MRM) mass spectrometry. The biological samples are treated with formaldehyde containing agents / fixatives including formalin-fixed tissue / cells, formalin-fixed / paraffin embedded (FFPE) tissue / cells, FFPE tissue blocks and cells from those blocks. A protein digest is prepared from a biological sample and the ENT1, ERCC1, FOLR1, RRM1, TUBB3, TOPO1, and / or TOPO2A proteins are quantitated in the digest by quantitating in the protein sample one or more of the peptides described by the method of SRM / MRM mass spectrometry.
Owner:EXPRESSION PATHOLOGY
Tumor repair gene mutation fluorescent real time PCR detection method and reagent system
InactiveCN101266207AMicrobiological testing/measurementMaterial analysis by optical meansOligonucleotide primersFluorescence
The invention relates to a real-time PCR detecting method of tumor-associated gene mutation and reagent system, belonging to medicine curative effect detecting technical field. The tumour repair genes XRCC3, ERCC1 and ERCC2 associated with the tumour individualized theraphy are used as target genes to detect, and the customed designed special oligonucleotide primer sequence, hydrolysis probe and Taqman-MGB detection agent system are used and using real-time quantitative PCR method, therefore the mutation of special site of the tumor-associated gene is detected wherein the agent system is just the real-time quantitative PCR agent system comprising any oligonucleotide primer sequence and probe. The method can be used for effect detection of the clinical individualized medication scheme.
Owner:SHANGHAI PULMONARY HOSPITAL
Primer group, reagent and/or kit and system for detecting lung cancer chemotherapy related genes, and application
ActiveCN109517900AClustering is clearImprove accuracyMicrobiological testing/measurementDNA/RNA fragmentationCYP2C8Biology
The invention relates to a primer group, reagent and / or kit and system for detecting lung cancer chemotherapy related genes, and application. The primer group contains primers for detecting gene mutation of ERCC1, MTHFR, GSTP1, XRCC1, DYNC2H1, ABCB1, CYP2C8*3, TP53, NQO1, CBR3, SOD2, CYP2C19, UGT1A1*6, TYMS, NT5C2 and CDA. The primer group, the reagent and / or the kit, the system and the application have the advantages of high accuracy, high specificity, high sensitivity, good precision and the like, and meanwhile, further have the advantages of sample saving, short detection period, easy operation, convenient analysis and the like.
Owner:SIMCERE DIAGNOSTICS CO LTD +2
Kit for detecting expression level of human ERCC1 (excision repair cross complementation 1) through PCR (polymerase chain reaction) method
InactiveCN103451303AStrong specificityEliminate non-experimental differencesMicrobiological testing/measurementFluorescence/phosphorescencePhosphateSynchronous fluorescence
Owner:JIANGSU KANGKE BIOTECH
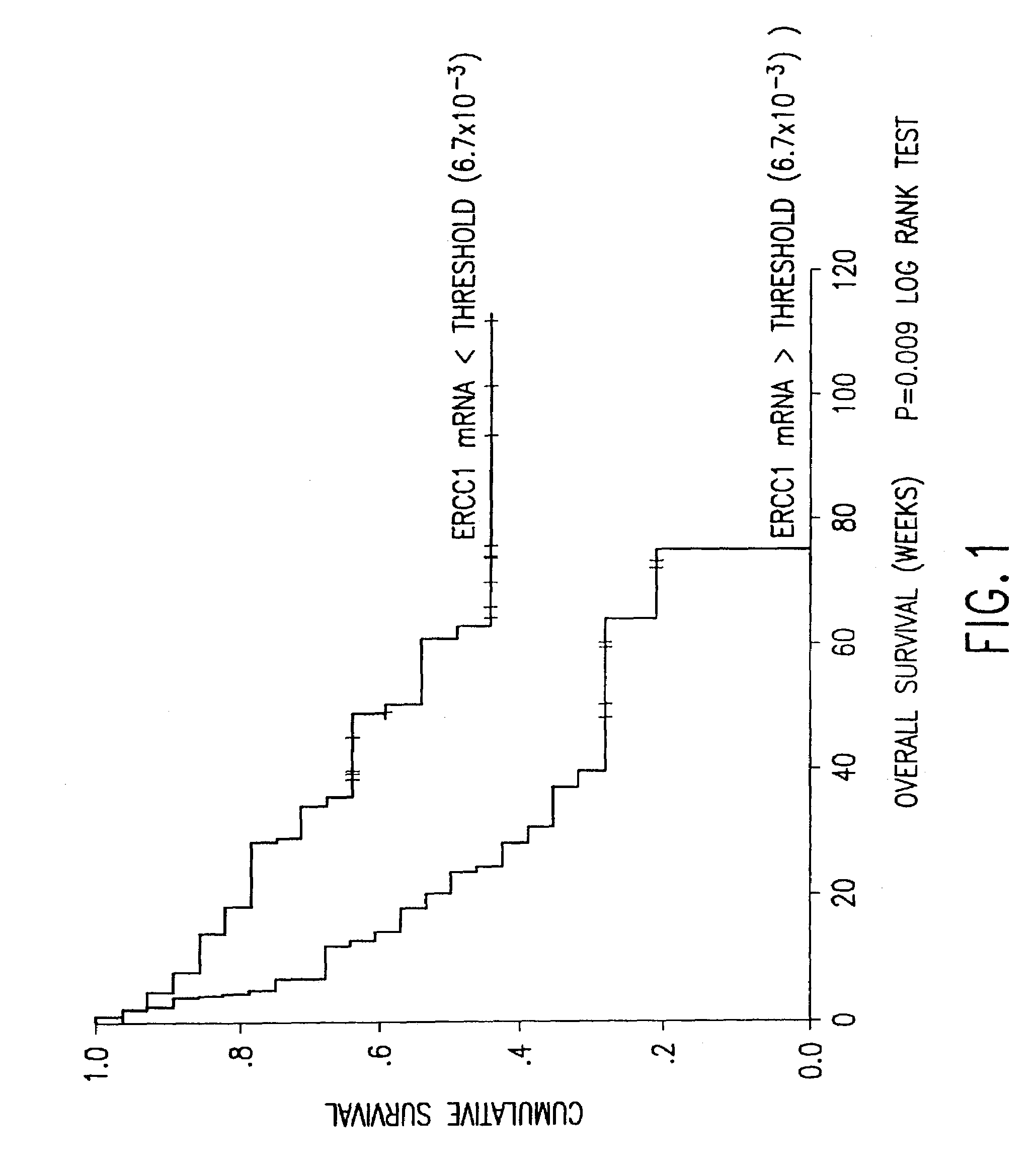
Ercc1 expression in predicting response for cancer chemotherapy
InactiveUS20090215090A1Efficient use ofEasy to useImmunoglobulins against enzymesMaterial analysisFormalin fixed paraffin embeddedERCC1
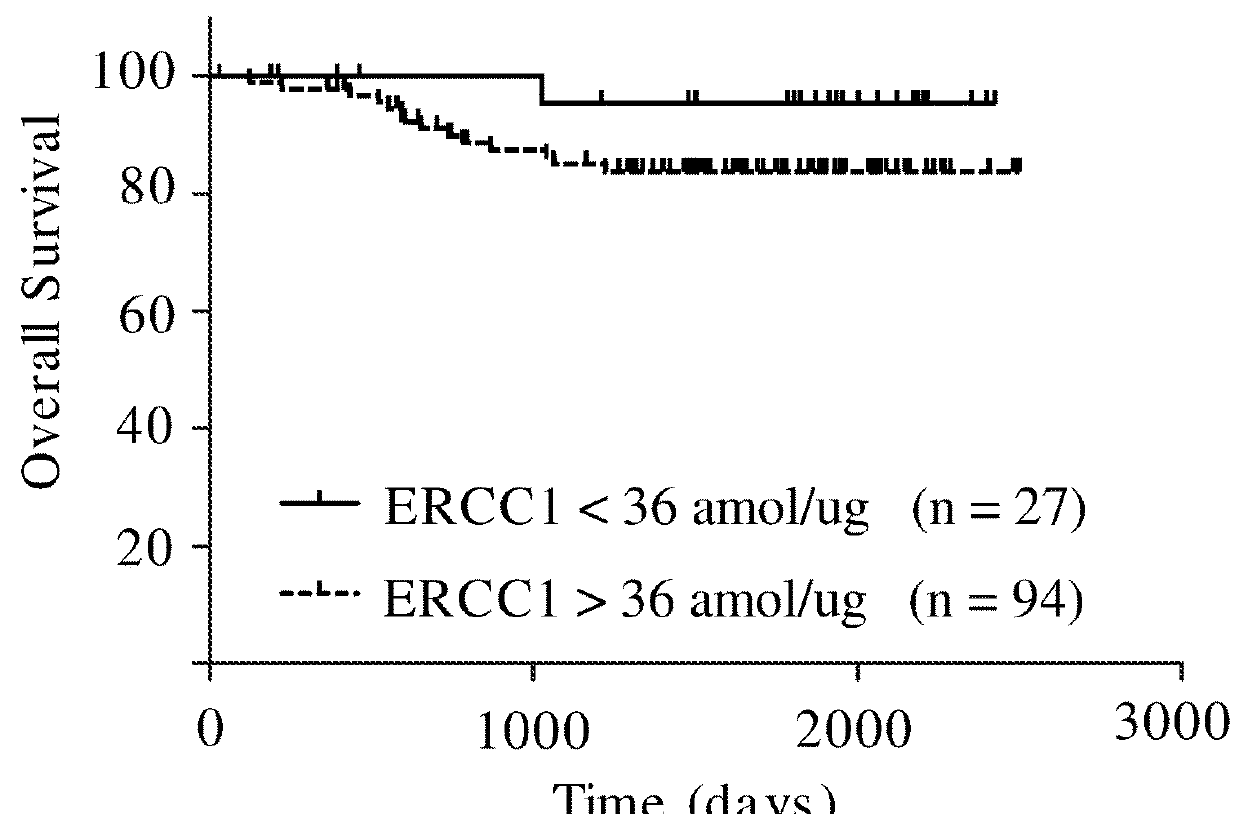
The present invention concerns an in vitro method for detecting the susceptibility of a tumor cell to a chemotherapy, said method comprising the step of the measurement of the ERCC1 protein by immunohistochemistry in a formalin-fixed paraffin-embedded tumor sample.
Owner:INSTITUT GUSTAVE ROUSSY
Multi-gene detection kit for selection of chemotherapy regimens
ActiveCN103882139AEasy to analyzeReliable analysisMicrobiological testing/measurementDPYDReference genes
The invention relates to a multi-gene detection kit for the selection of chemotherapy regimens. The multi-gene detection kit comprises a mixture of RT (reverse transcriptase primer) primers and a mixture of PCR (Polymerase Chain Reaction) amplification primers, wherein the mixture of RT primers and the mixture of PCR amplification primers comprise each RT primer and each PCR amplification primer based on genetic groups, reference genes and an internal standard substance of the reaction respectively, also comprise random primers, the genetic groups comprise RRM1, TOP2A, ERCC1, TYMS, TUBB3, TOP1, PTEN, HER2, DPYD and EGFR; the reference genes comprise ACTB, GAPDH and B2M; the internal standard substance of the reaction is KAN-rRNA. The multi-gene detection kit for the selection of chemotherapy regimens, which is disclosed by the invention, can be used for simultaneously detecting the expression levels of multiple genes closely related to anti-tumor medicines systematically, and is easy to use, good in accuracy, high in detection efficiency and can be well used to guide chemotherapy medication and select chemotherapy regimens.
Owner:南昌市赛尔医药科技有限公司
Methods of Treating Lung Cancer by Predicting Responders to Cisplatin-Pemetrexed Combination Therapy
ActiveUS20180177825A1Inhibition formationHeavy metal active ingredientsOrganic active ingredientsPulmonary neoplasmsCisplatin
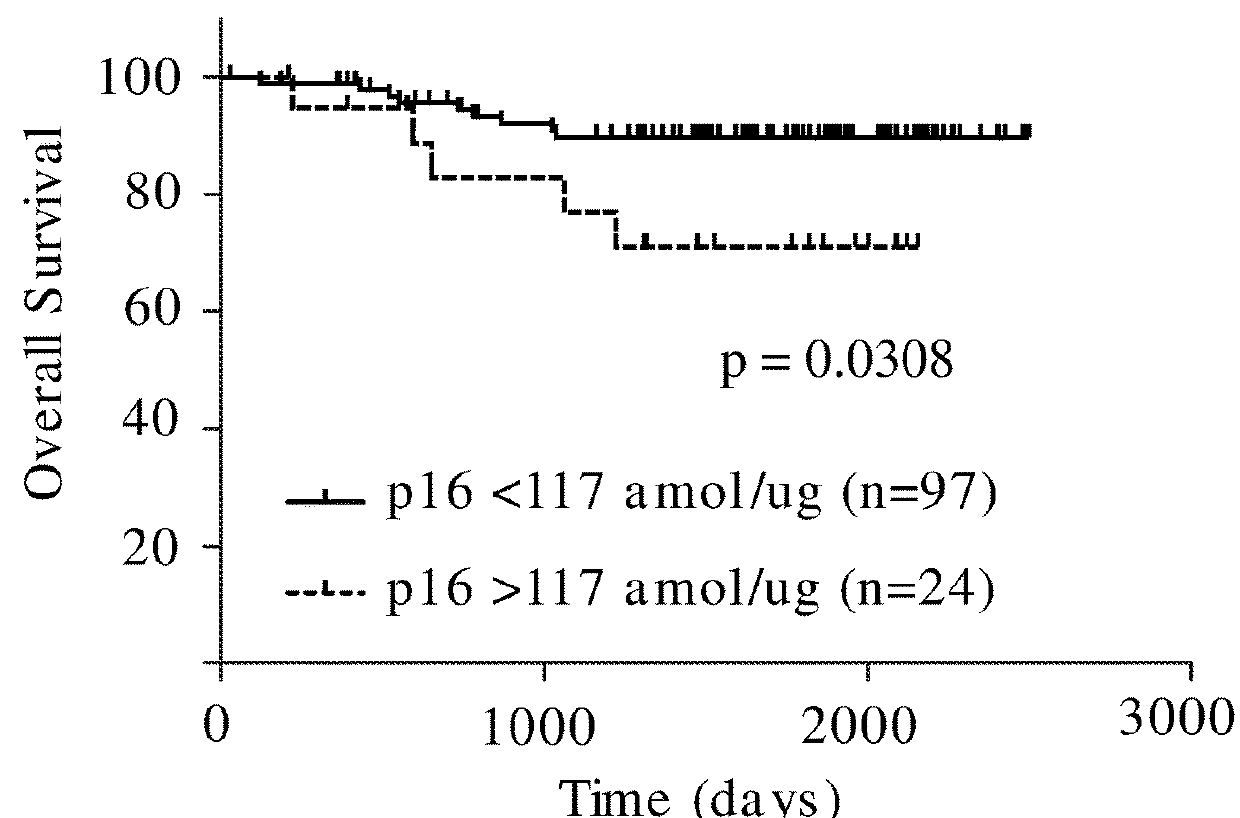
Methods are provided for identifying whether a lung tumor will be responsive to treatment with the combination of the therapeutic agents cisplatin and pemetrexed. Specified ERCC1, TS, p16, and FRα fragment peptides are precisely detected and quantitated by SRM-mass spectrometry directly in lung tumor cells collected from lung tumor tissue that was obtained from a cancer patient and compared to reference levels in order to determine if the lung cancer patient will positively respond to treatment with the combination of cisplatin and pemetrexed therapeutic agents.
Owner:EXPRESSION PATHOLOGY
Anti ERCC1 monoclonal antibody 2E12 and application
ActiveCN102786597AStrong specificityImprove accuracyMicroorganism based processesTissue cultureExcision repair cross-complementingWilms' tumor
The invention relates to a monoclonal antibody 2E12 capable of specifically bonding excision repair cross complementing action group 1 (ERCC1) protein, generated hybridoma of the monoclonal antibody, a composition of the monoclonal antibody, and a usage method and an application of the monoclonal antibody. The monoclonal antibody 2E12 can overcome the non-specificity problem of ERCC1 monoclonal antibody 8F1, can be used for prognosis of tumors such as non-small cell lung cancer, ovary cancer, breast cancer, renal adenocarcinoma, endometrial carcinoma, and can realize the curative effect prediction for auxiliary chemotherapy with platinum containing chemotherapeutics.
Owner:北京中杉金桥生物技术有限公司
Primer and method for simultaneously detecting XRCC1, ERCC1 and GSTP1 gene polymorphisms
InactiveCN104988224AStrong specificityImprove accuracyMicrobiological testing/measurementDNA/RNA fragmentationSpecific detectionMedicine
The invention belongs to the technical field of biological detection, and provides a primer for simultaneously detecting XRCC1, ERCC1 and GSTP1 gene polymorphisms. The primer comprises a PCR amplification primer and a SNaPshot PCR primer. The primer can achieve specific detection on XRCC1, ERCC1 and GSTP1 gene polymorphisms, causes no cross reaction and is good in accuracy.
Owner:GUANGZHOU KINGMED DIAGNOSTICS CENT
Reagent kit for detecting lawful age females synthetic disease genetic susceptibility
InactiveCN101275911AMicrobiological testing/measurementChemiluminescene/bioluminescenceDiseaseFluorescence
The present invention discloses a reagent kit which detects the genetic susceptibility of the synthetic disease of adult woman. The reagent kit comprises a specific primer pair and a specific fluorescent probe pairs which simultaneously detects the SNP polymorphic genotype on the genes of CYBA, CAT, LPL, LEP, ADIPOQ, SOD3, IL3, NOS3, CTLA4, ENOS, ESR2, ERCC1, MTHFR, MS4A2, XRCC1, ALDH2, TNFA, MTR, CCL5, COMT, GSTP1, PPARG, PARP1, OPG, VDR, PON1, ABCA1, MTRR, ERCC2, AT1R, AGT, CCND1, UCP2, ADH2, ADBR2, APOB, CYP11B2, CYP1A1, CYP2E1, CETP, NQO1, IL6, CYP2A13, CBS, a routine component which is used for the fluorescent quantitative PCR testing and the like. The reagent kit of the invention evaluates the genetic susceptibility of the synthetic disease of adult woman through simultaneously detecting the mononucleotide polymorphism site genotype which is closely linked to the genetic susceptibility of the synthetic disease of adult woman.
Owner:XINBAXIANG SHANGHAI MOLECULAR MEDICAL TECH SHANGHAI
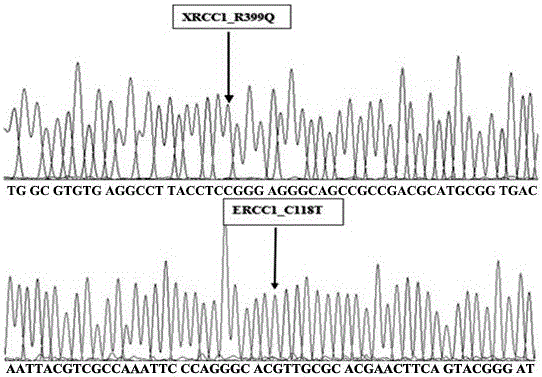
Nucleic acid test strip method for detecting polymorphism of 118th codon of ERCC1 (excision repair cross complement)
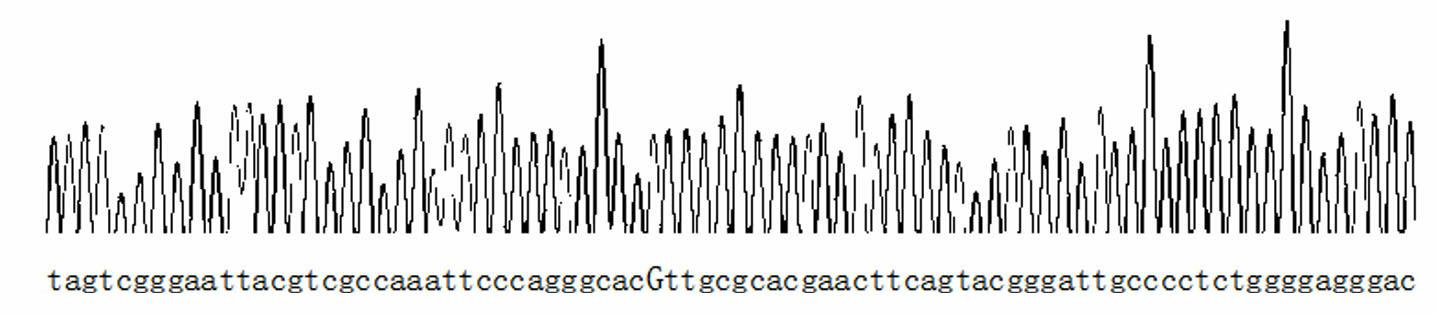
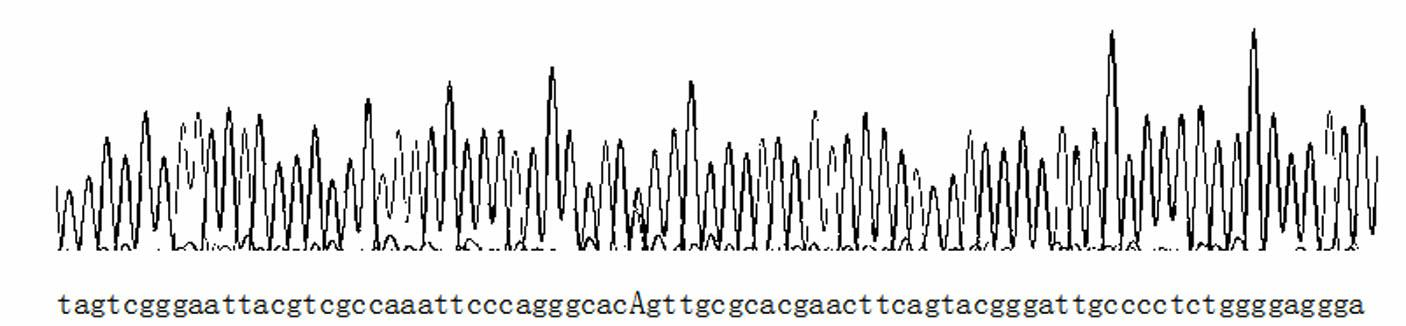
InactiveCN103820526AImprove featuresIncreased sensitivityMicrobiological testing/measurementMaterial analysisNucleic acid detectionERCC1
The invention is a technology for performing allele specific amplification by using a labeled primer on the basis of PCR (polymerase chain reaction) amplification of a target template, and eventually detecting the 118th codon of an ERCC1 (excision repair cross complement) by a nucleic acid test strip method. The main detection principle is as follows: according to immunological specific binding of an antigen and a specific antibody thereof, a nucleic acid fragment is fixed on the nucleic acid detection test strip, and then according to color development of visual colorimetric latex particles on the test strip, a result is detected. In the technology for detecting polymorphic genotypes of the 118th codon of the ERCC1 by the PCR & AS-PCR nucleic acid test strip method, a specific PCR amplification primer for PCR amplification, an allele-specific primer with a biotin label at 5' end, and a specific probe with an FITC (fluorescein isothiocyanate) label at 3' end are mainly used.
Owner:SUZHOU YOUBEIWO BIOTECH
Reagent kit for detecting man disease genetic susceptibility
The present invention provides a kit for detecting genetic predisposition of adult male syndrome. The kit includes specific primer pairs and specific fluorescent probe pairs for detecting 48 SNP polymorphism genotypes on the genes LPL, TNF-alpha, LEP, ADIPOQ, SOD3, IL3, CTLA4, ENOS, ESR2, ERCC1, MTHFR, MS4A2, XRCC1, ALDH2, TNFA, MTR, CCL5, COMT, GSTP1, PPARG, PARP1, OPG, VDR, PON1, ABCA1, MTRR, ERCC2, AT1R, AGT, CCND1, UCP2, ADH2, ADBR2, APOB, CYP11B2, CYP1A1, CYP2E1, CETP, NQO1, IL6, CYP2A13 at the same time, and normal components for FQ-PCR detection. The kit of the invention evaluates the genetic predisposition of adult male syndrome by detecting the genotypes of the 48 SNP polymorphism sites at the same time which is tightly relative to the genetic predisposition of adult male syndrome.
Owner:XINBAXIANG SHANGHAI MOLECULAR MEDICAL TECH SHANGHAI
Kit for platinum drug medication guidance
InactiveCN104862381AImprove stabilityImprove throughputMicrobiological testing/measurementDNA/RNA fragmentationPlatinumAgricultural science
The invention discloses a kit for platinum drug medication guidance. The invention protects a primer group. The primer group comprises a primer pair A, a primer pair B and a primer pair C. The primer pair A comprises a single chain DNA molecule shown in the sequence 1 and a single chain DNA molecule shown in the sequence 2. The primer pair B comprises a single chain DNA molecule shown in the sequence 3 and a single chain DNA molecule shown in the sequence 4. The primer pair C comprises a single chain DNA molecule shown in the sequence 5 and a single chain DNA molecule shown in the sequence 6. The invention also protects a kit for identification of C118T point mutation of an ERCC1 gene, R194W point mutation of a XRCC1 gene and I105V point mutation of a GSTP1 gene. The kit comprises the primer group. The kit can be used for detecting three platinum drug sensitivity-related SNP sites, can be further used for platinum drug individual use guidance and improves clinical treatment specificity and predictability.
Owner:北京奥维森基因科技有限公司
Reagent kit for detecting children disease genetic susceptibility
The present invention provides a kit of detecting genetic predisposition of children syndrome. The kit includes specific primer pairs and specific fluorescent probe pairs for detecting 28 SNP polymorphism genotypes on the genes NOS3, CTLA4, ENOS, ERCC1, ERCC2, MTHFR, MTRR, MTR, MS4A2, XRCC1, ALDH2, TNFA, CCL5, PARP1, OPG, VDR, PON1, AGT, CCND1, UCP2, ADH2, ADBR2, APOB, CYP11B2, CYP1A1, CYP2E1, CETP, NQO1 at the same time, and normal components for FQ-PCR detection. The kit of the invention evaluates the genetic predisposition of children syndrome by detecting the genotypes of the 28 SNP polymorphism sites at the same time which is tightly relative to the genetic predisposition of children syndrome.
Owner:XINBAXIANG SHANGHAI MOLECULAR MEDICAL TECH SHANGHAI
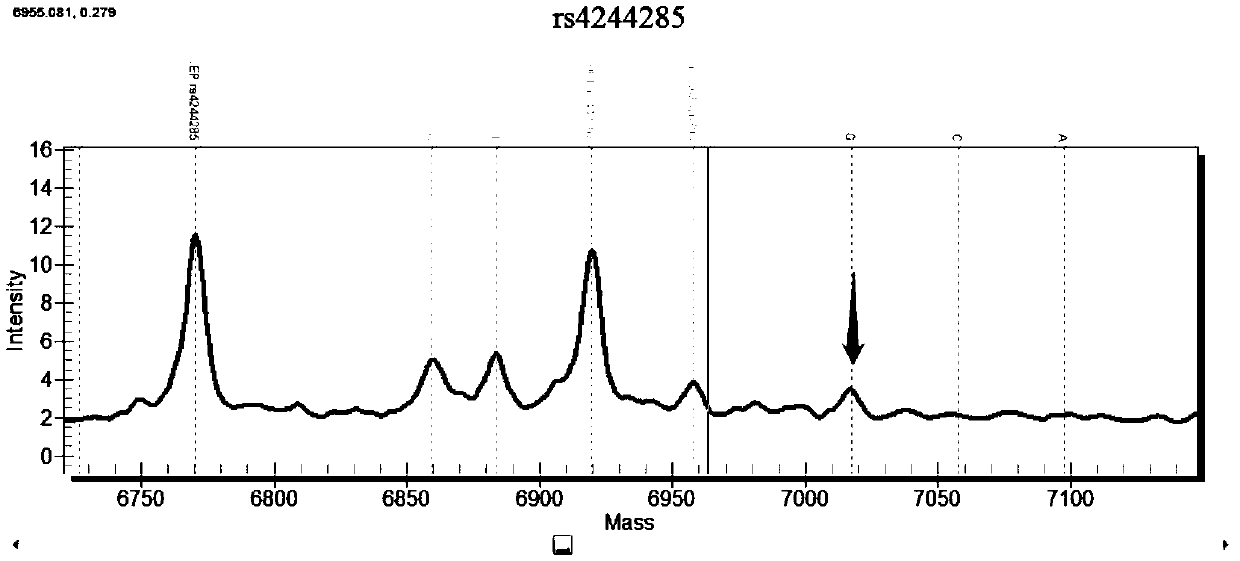
Fluorescent polarization based homogeneous phase detection method of single nucleotide polymorphism of codon118 of ERCC1 (excision repair cross-complementing 1) gene
InactiveCN102676661AThe method steps are simpleEasy to operateMicrobiological testing/measurementDNA/RNA fragmentationERCC1Nucleotide
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
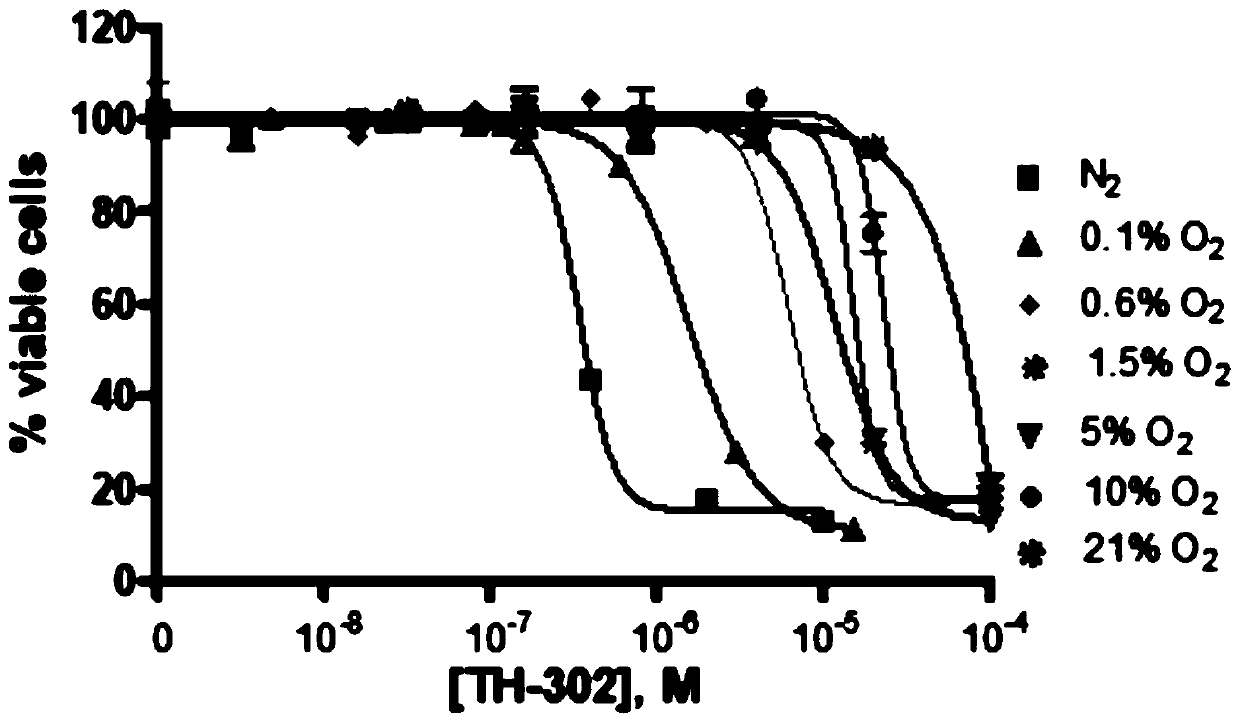
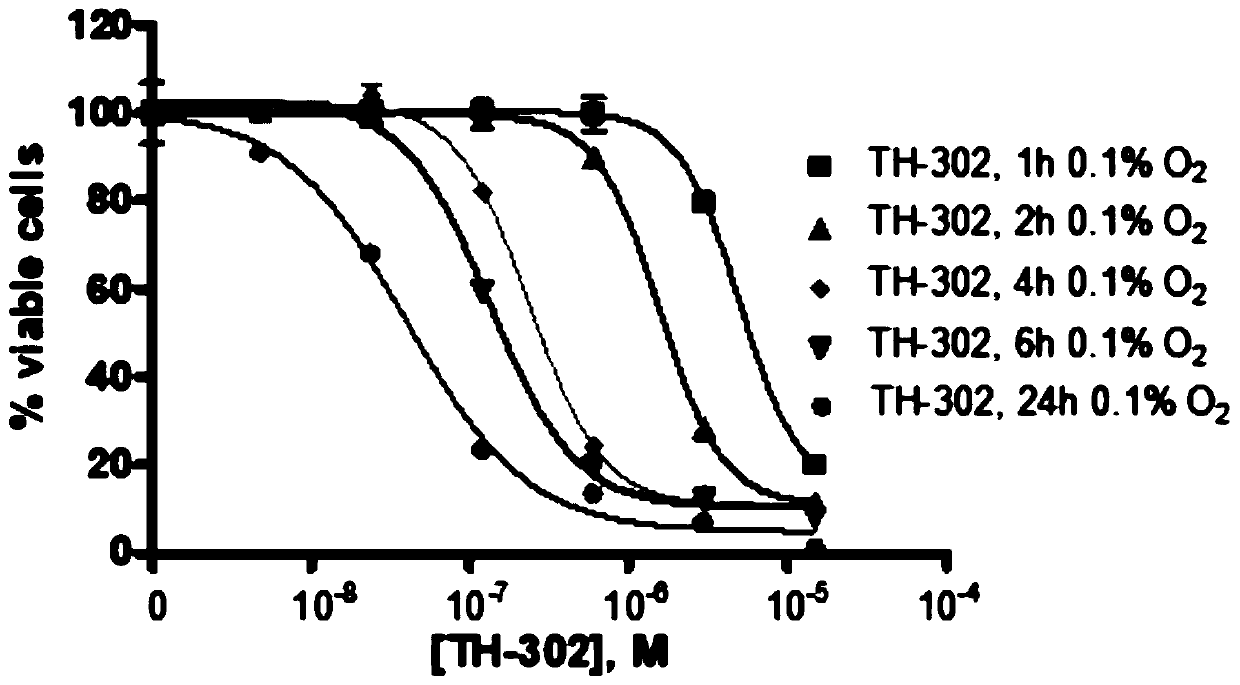
Medical use of evofosfamide in anti-cancer
Evofosfamide (TH-302) or an analog thereof has a specific inhibitory effect on a cell having a specific gene mutation, in particular on a DNA repair-damaged cell. The cell or tissue has at least one or more gene mutations in BRCA1, BRCA2, FANCD1, FANCD2, ATM, ATR, CHEK1, CHEK2, CTP, BARD1, BRIP1, PALB2, RAD51D, RAD51C, RAD52, RAD54, RAD55, RAD57, FAM175, NBN, Rad50, MER11, p53, NBS1, XRS2, XRCC2,XRCC3, ERCC1, ERCC2, ERCC3, ERCC4, XRCC1, Ku80, MHS6, MGMT, PARP, and ERCC5. For this, a medical use of evofosfamide (TH-302) or an analog thereof in treating tumors or cancer in a cancer patient having the specific gene mutation(s) is provided.
Owner:SHENZHEN ASCENTAWITS PHARM TECH CO LTD
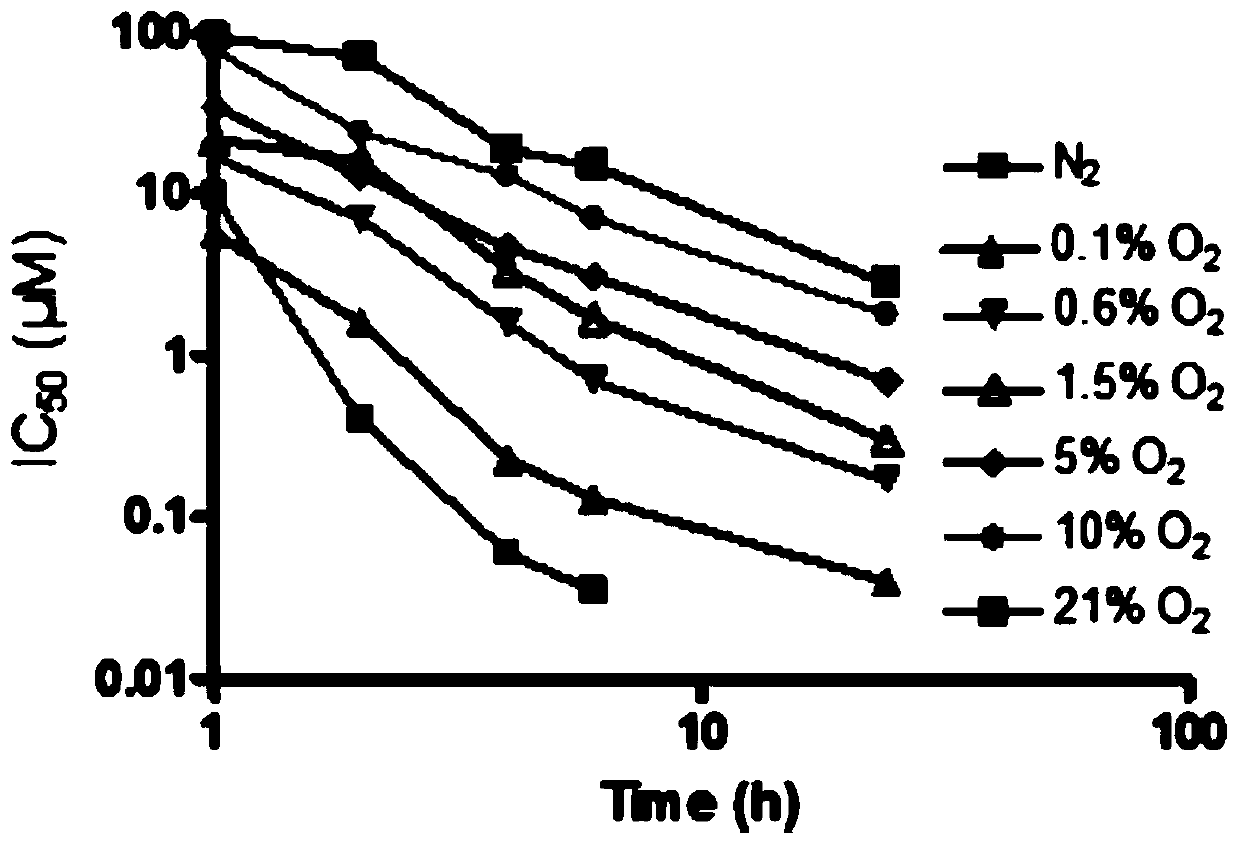
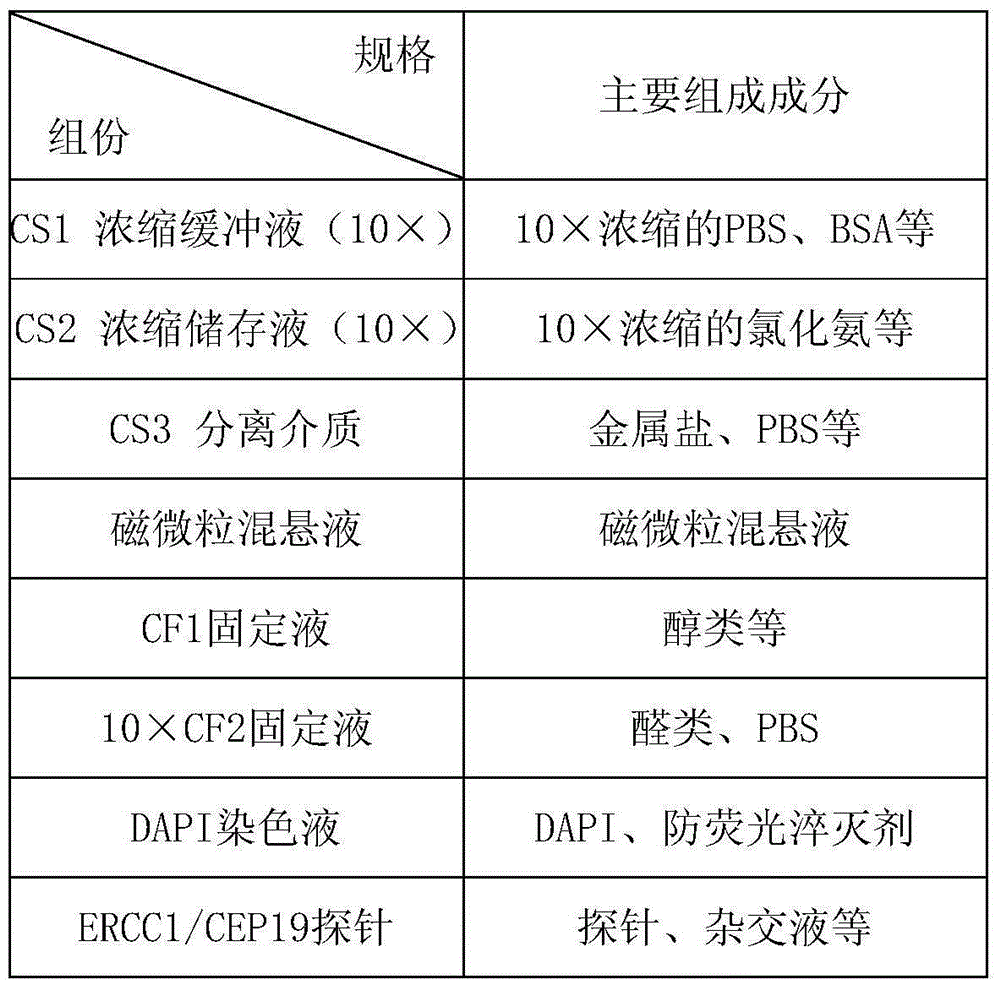
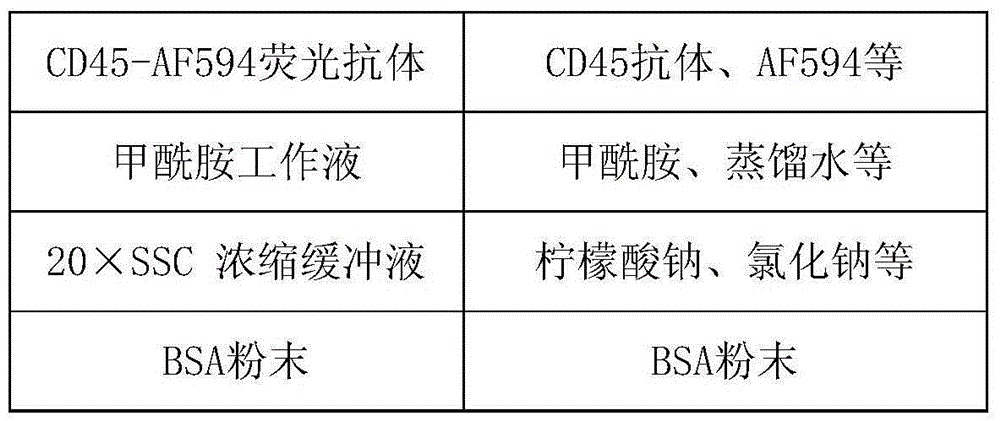
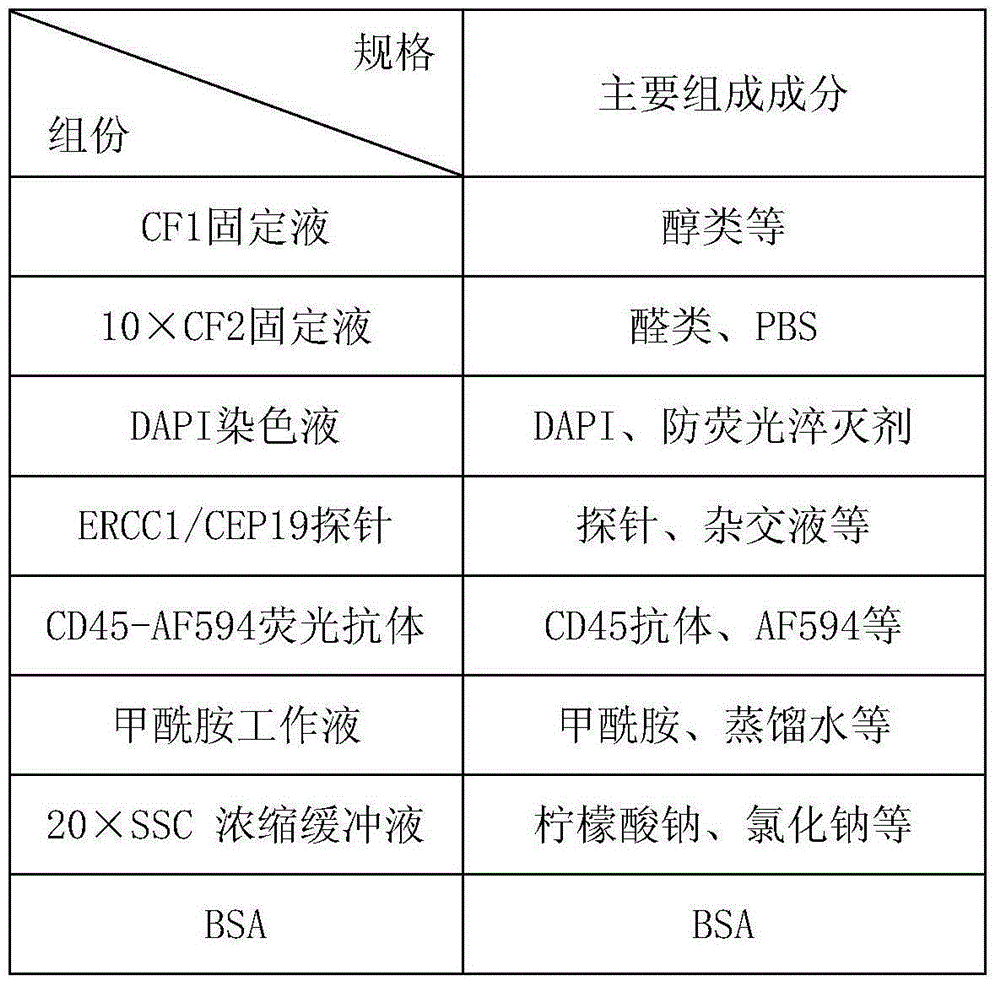
Method for detecting ERCC1/CEP19 gene status based on rare cell and correlated kit
The invention relates to a method for detecting ERCC1 / CEP19 (excision repair cross-complementing 1) gene status based on a rare cell and a correlated kit. The method comprises the following steps: step 1, acquiring a blood sample or a body fluid sample, wherein the sample contains a mixed cell population of circulating tumor cell (CTC) or other rare cells and leukocyte; step 2, using a buffer to dilute the sample, removing blood plasma, and recovering CTC or other rare cells; step 3, performing lysis by using a erythrocyte lysate, removing erythrocyte, and recovering CTC or other rare cells; step 4, uniformly mixing a magnetic particle coated with an anti-human-leucocyte correlated antigen-antibody with the recovered cell and performing incubation, so as to form a magnetic particle-leucocyte mixture through combination; step 5, centrifuging to separate the magnetic particle-leucocyte mixture from other cells, so as to obtain a mixed cell population containing CTC or other rare cells and a few of leucocytes; and step 6, using immunofluorescence cytochemistry and fluorescence in-situ hybridization combined method to determine the state of ERCC1 / CEP19 and also identify CTC and other rare cells.
Owner:北京莱尔生物医药科技有限公司
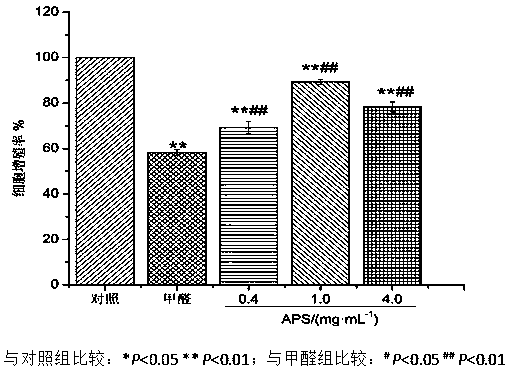
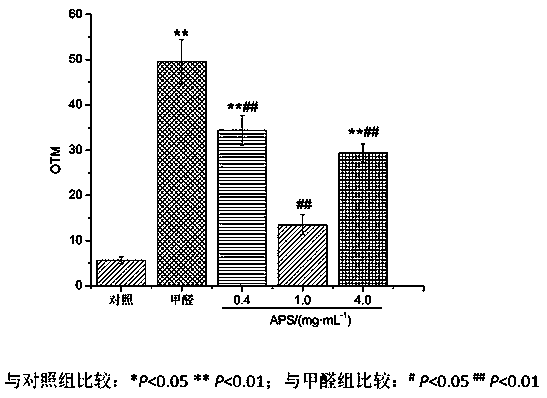
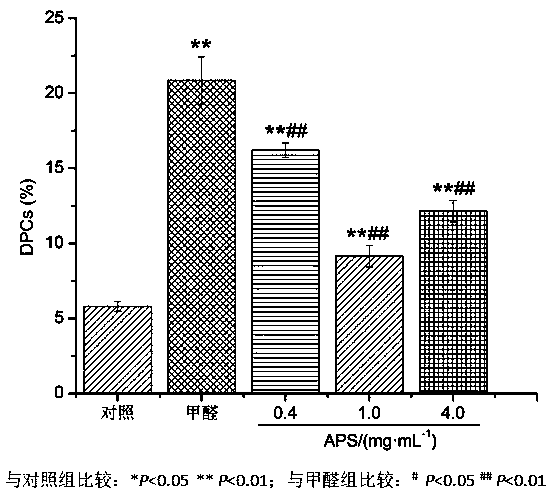
New application of astragalus polysaccharide
InactiveCN110179813AIncrease proliferative activityReduced DNA Fragmentation EffectsOrganic active ingredientsAntinoxious agentsProliferation activityLeukemia
The invention discloses a new application of astragalus polysaccharide, and belongs to the technical field of biology medicine. A basis is provided for preventing and treating myoblastosis through radix astragali. The inventor of the invention finds that the astragalus polysaccharide can obviously reinforce the proliferation activity of human BM-MSCs in formaldehyde environment, and can reduce DNAfragmentation and DPCs formation of human BM-MSCs induced by formaldehyde so as to have good protection effects. The astragalus polysaccharide can promote DNA injury repair factors of XPA, XPC, ERCC1, RPA1 and RPA2 mRNA and protein expression, and improve DNA damage repair capacity. The inventor finds that the astragalus polysaccharide can reinforce the proliferation activity of human BM-MSCs inthe formaldehyde environment for the first time, can reduce the DNA fragmentation effects of the human BM-MSCs in the formaldehyde environment and can reduce the DPCs formation of the human BM-MSCs inthe formaldehyde environment.
Owner:GANSU UNIV OF CHINESE MEDICINE
Expression of isoform 202 of ERCC1 for predicting response to cancer chemotherapy
InactiveUS9702875B2Practical to useEfficient use ofMicrobiological testing/measurementDisease diagnosisERCC1Tumor cells
An in vitro method for detecting the susceptibility of a tumor cell to a chemotherapy is disclosed. The method includes the step of measuring the expression level of the isoform 202 of the ERCC1 protein.
Owner:INSTITUT GUSTAVE ROUSSY
Anti ERCC1 monoclonal antibody 2E12 and application
ActiveCN102786597BStrong specificityImprove accuracyMicroorganism based processesTissue cultureExcision repair cross-complementingWilms' tumor
The invention relates to a monoclonal antibody 2E12 capable of specifically bonding excision repair cross complementing action group 1 (ERCC1) protein, generated hybridoma of the monoclonal antibody, a composition of the monoclonal antibody, and a usage method and an application of the monoclonal antibody. The monoclonal antibody 2E12 can overcome the non-specificity problem of ERCC1 monoclonal antibody 8F1, can be used for prognosis of tumors such as non-small cell lung cancer, ovary cancer, breast cancer, renal adenocarcinoma, endometrial carcinoma, and can realize the curative effect prediction for auxiliary chemotherapy with platinum containing chemotherapeutics.
Owner:北京中杉金桥生物技术有限公司
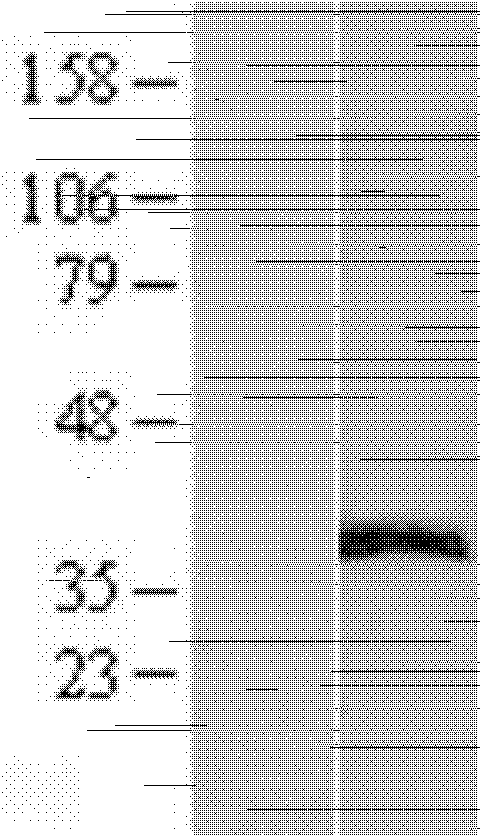
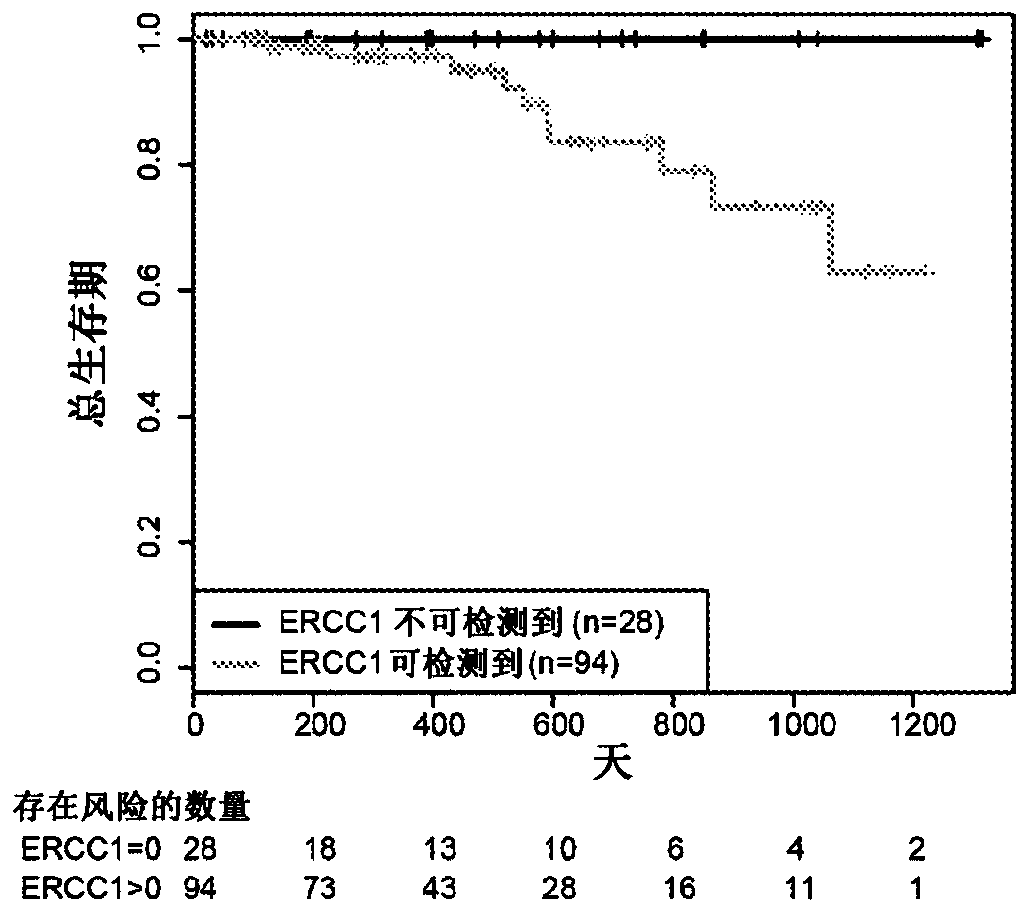
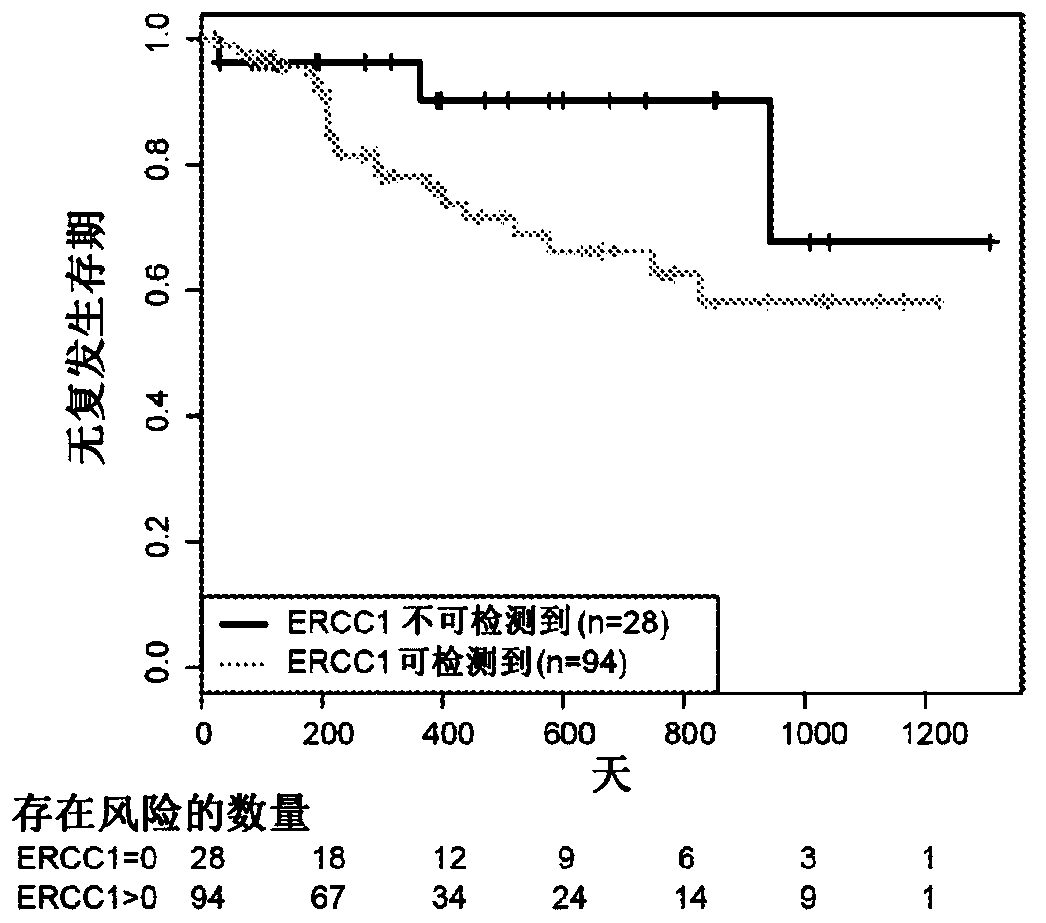
Ercc1 and other markers for stratification of non-small cell lung cancer patients
Proteomics data are employed to predict treatment outcome for cisplatin / pemetrexed therapy of NSCLC. Notably, ERRC1 can be used as a binary marker where protein presence or absence is measured by massspectroscopy, and additional proteins may serve as further predictive markers, particularly for prediction of PFS.
Owner:NANTOMICS LLC
A multiple gene detection kit related to anti-tumor drugs
ActiveCN103882138BEasy to analyzeReliable analysisMicrobiological testing/measurementERCC1Chemotherapy combinations
The invention relates to a multiple-gene detecting kit related to antitumor drugs. The multiple-gene detecting kit comprises a mixture of RT (reverse transcription) primers and a mixture of PCR (polymerase chain reaction) amplification primers, wherein each RT primer and each PCR amplification primer based on genetic groups, reference genes and a reaction internal label are respectively contained in each of the mixture of the RT primers and the mixture of the PCR amplification primers; the multiple-gene detecting kit is characterized in that the genetic groups comprise PTEN, EGFR, DPYD, HER2, RRM1, ERCC1, TUBB3, TOP1, TYMS and TOP2A; the reference genes comprise ACTB, GAPDH and B2M; the reaction internal label is KAN-rRNA (ribosomal RNA). The multiple-gene detecting kit related to antitumor drugs disclosed by the invention can be used for systemically detecting the expression level of a plurality of genes closely related to the antitumor drugs by one step, is simple and convenient to use, good in accuracy and high in detecting efficiency, and can be used for guiding chemotherapy drugs and selecting chemotherapy regimens very well.
Owner:南昌市赛尔医药科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com