Anti ERCC1 monoclonal antibody 2E12 and application
A monoclonal antibody, antibody technology, applied in anti-enzyme immunoglobulin, biochemical equipment and methods, instruments, etc., can solve problems affecting the specificity of immunohistochemical results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] Embodiment 1, construction of ERCC1 recombinant expression plasmid
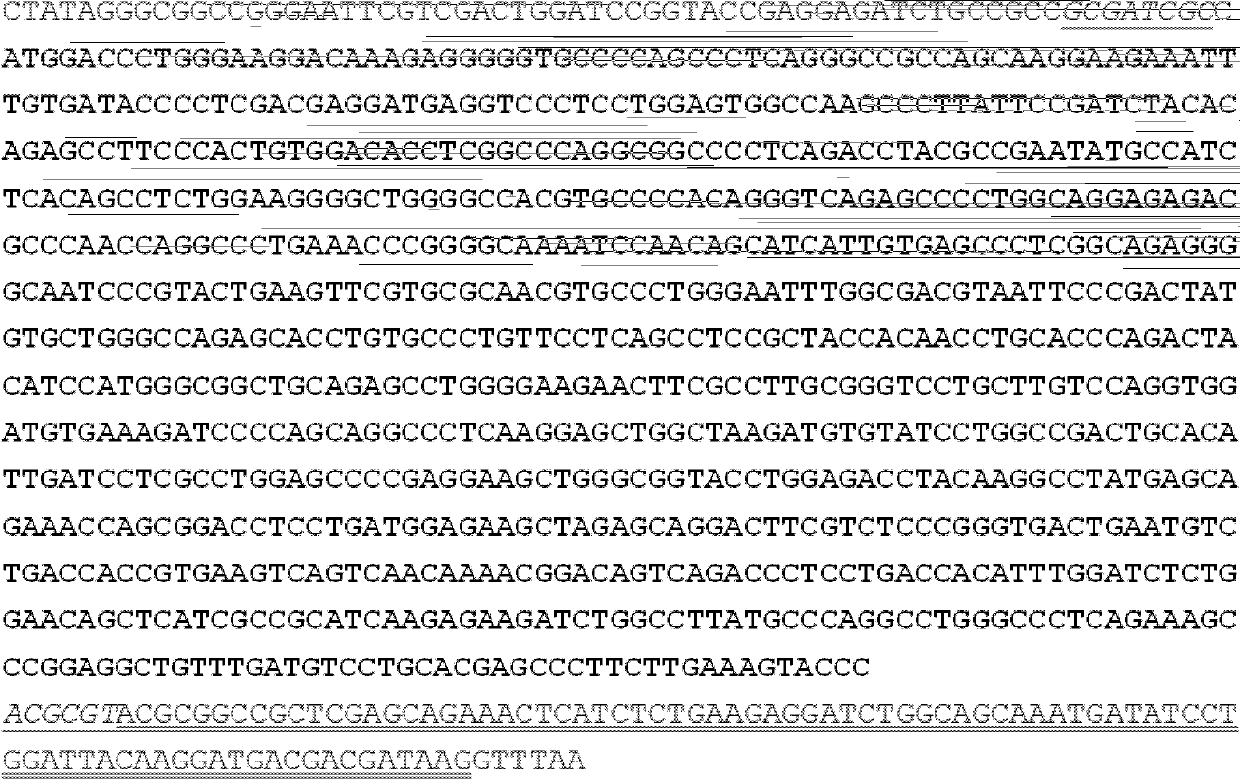
[0069] The plasmid BC0089302 (containing ERCC1ORF 894bp) obtained from ATCC was used as a template, and 5'-GAGAGCGATCGCCATGGACCCTGGGAAGGACAAAG-3' (SEQ ID NO: 2) was used as a forward primer (the 5' end of which was introduced into the restriction endonuclease SgfI site GCGATCGC) , 5'-CGAGCCCTTCTTGAAAGTACCCACGCGTGAGA-3' (SEQ ID NO: 3) is the reverse primer (the 5' end introduces the restriction endonuclease MluI site ACGCGT), obtains ERCC1ORF through PCR amplification, and introduces it on both sides respectively Recognition sites for restriction enzymes SgfI and MluI. According to the manufacturer's instructions, using restriction endonucleases SgfI and MluI (ABI company), the PCR product was cloned into the expression vector pCMV6-Entry (derived from origene company), thereby constructing the recombinant plasmid pCMV6-ERCC1 expressing ERCC1, the recombinant The plasmid carries Myc tag and Flag tag do...
Embodiment 2
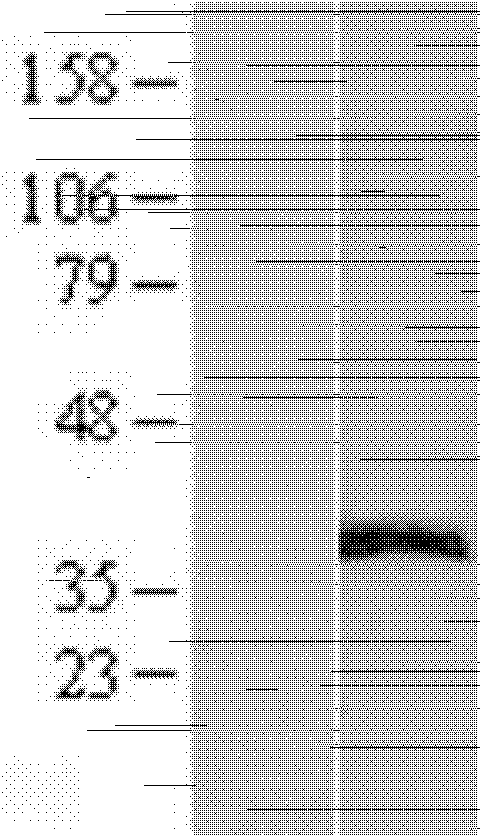
[0070] Example 2, Expression and Purification of ERCC1 Recombinant Antigen
[0071] Expression of ERCC1 recombinant protein
[0072] Using methods well known to those skilled in the art, the pCMV6-ERCC1 recombinant plasmid obtained in Example 1 was transfected into HEK293T cells. Briefly, at 37 °C in 5% CO 2 In the incubator (Thermo Company), HEK293T cells (derived from ATCC) were cultured in DMEM medium (Thermo Fisher Company) supplemented with newborn bovine serum and gentamycin until confluent, and then passaged at a ratio of 1:3. Continue culturing in a 10cm petri dish (Corning); take 7.5ml DMEM medium (without serum and antibiotics) into a 50ml tube, add 300ul PEI MegaTran1.0 (produced by origene) and mix well; then add 75μg pCMV6-ERCC1 recombinant Put the plasmid DNA into the above mixture, mix well and let it stand for 30 minutes; after standing still, take 515 μl into each culture dish and continue to culture the cells; 24 hours after transfection, add 2M sodium bu...
Embodiment 3
[0078] Example 3, Screening and Preparation of Monoclonal Antibody
[0079] animal immunity
[0080] Purified ERCC1 protein was used as antigen to immunize 6-8 week old BALB / c mice. The way of immunization is subcutaneous or intraperitoneal injection. For the first immunization, the antigen was emulsified with complete Freund's adjuvant, and the immunization dose was 50 μg per mouse. Two weeks later, the antigen was emulsified with incomplete Freund's adjuvant for the second immunization with a dose of 50 μg per mouse. After two immunizations, the mouse tail blood was taken to measure the titer of the serially diluted serum by ELISA method (coated with ERCC1 protein, goat anti-mouse antibody as the secondary antibody). Determine whether to further immunize the mice according to the measurement results, and select the mouse with the highest serum antibody titer for subsequent cell fusion.
[0081] cell fusion
[0082] The myeloma cells used were BALB / c-derived sp2 / 0 ce...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com