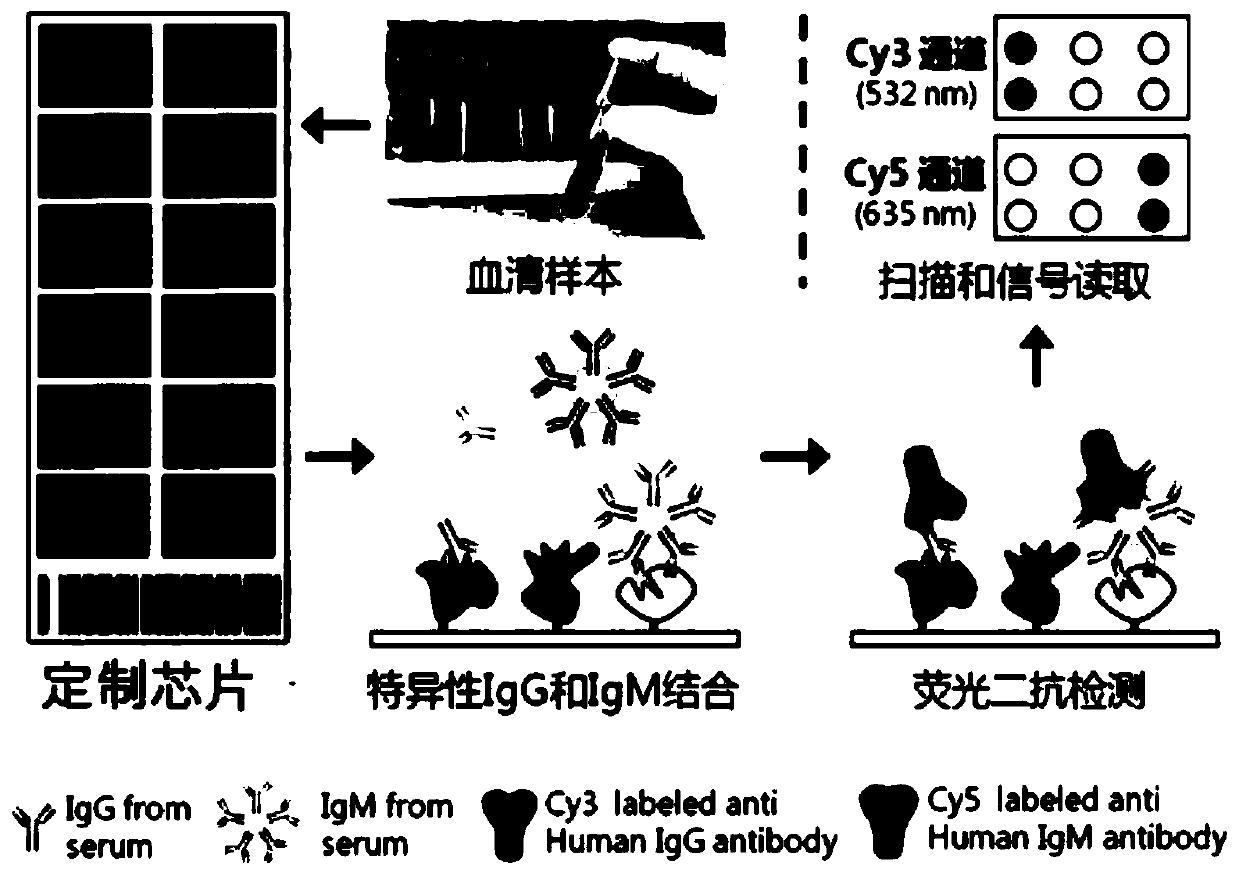
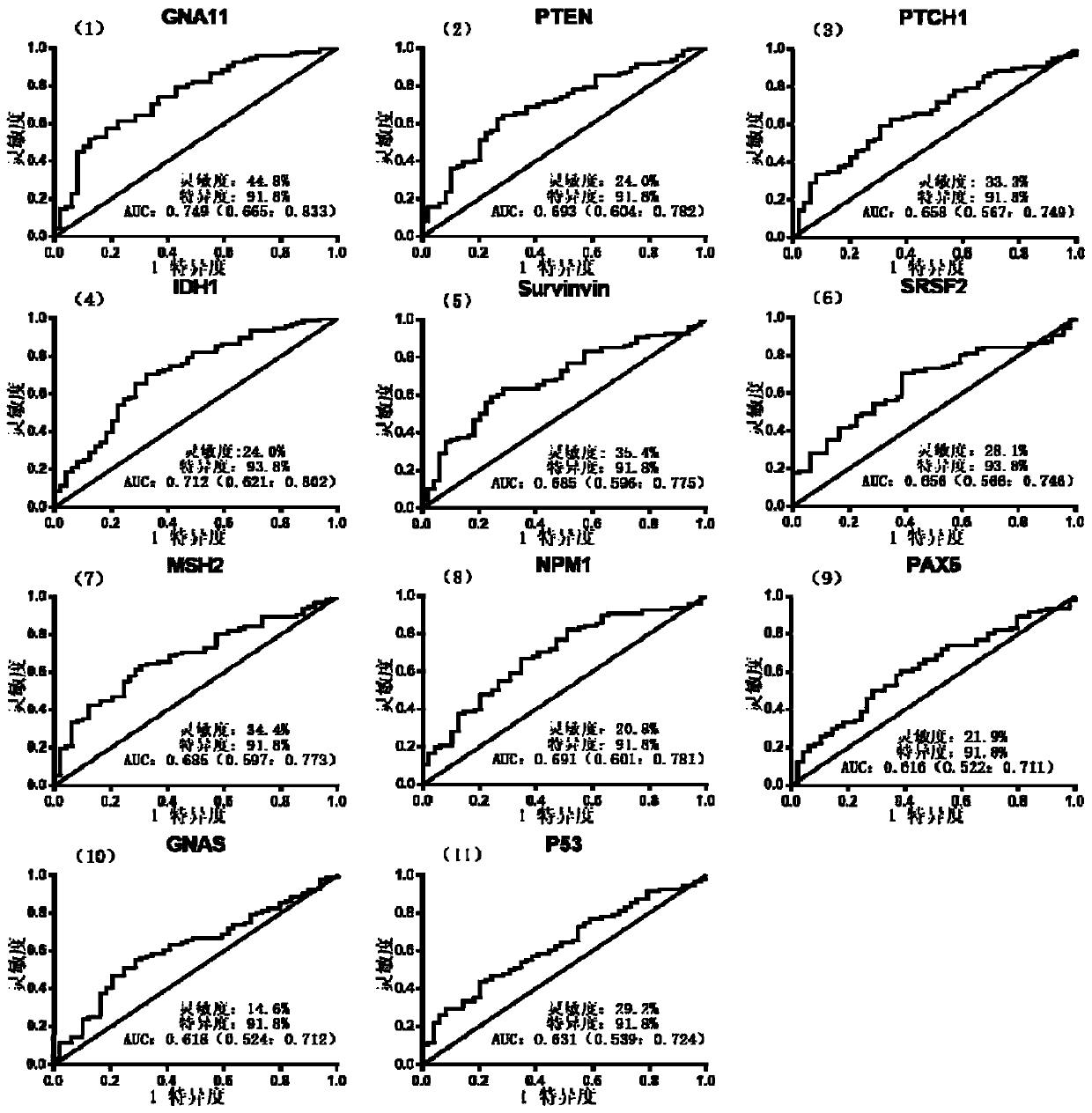
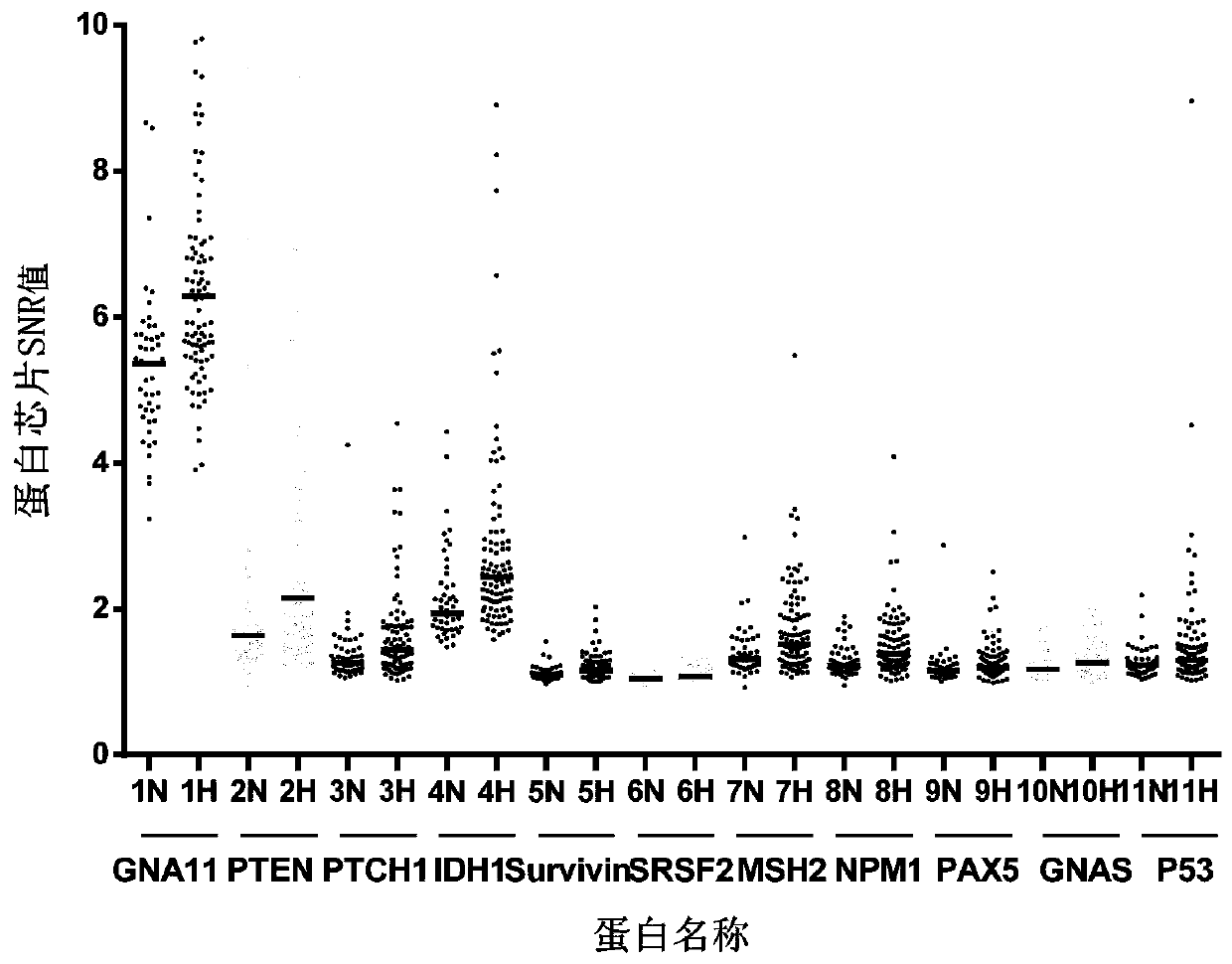
Combined detection serum marker for early screening and diagnosis of liver cancer, kit and detection method
A technology of combined detection of serum markers, applied in disease diagnosis, biological testing, measuring devices, etc., can solve problems such as unsatisfactory results, and achieve low cost, simple operation, and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] The combined detection serum markers used in this example for the early screening and diagnosis of liver cancer consist of proteins encoded by six genes including PTEN, PTCH1, IDH1, SRSF2, MSH2 and NPM1. Wherein the protein encoded by the PTEN gene has the amino acid sequence shown in SEQ ID NO.2, the protein encoded by the PTCH1 gene has the amino acid sequence shown in SEQ ID NO.3, and the protein encoded by the IDH1 gene has the amino acid sequence shown in SEQ ID NO.4 The amino acid sequence of the protein encoded by the SRSF2 gene has the amino acid sequence shown in SEQ ID NO.5, the protein encoded by the MSH2 gene has the amino acid sequence shown in SEQ ID NO.6, and the protein encoded by the NPM1 gene has the amino acid sequence shown in SEQ ID NO. . The amino acid sequence shown in 7.
Embodiment 2
[0048] The kit for the early screening and diagnosis of liver cancer in this example includes the combined detection serum markers of the above-mentioned Example 1, and is coated on a polyvinyl chloride concave-well plate. In addition, the kit also includes a certain amount of positive control serum, negative control serum, blocking solution, sample diluent, secondary antibody, secondary antibody diluent, washing solution, color developing solution and stop solution, and the positive control serum is ELISA The OD value of the experiment is higher and the corresponding antibody-positive serum is verified by Western Blot experiment. The negative control serum is the serum with the OD value of the ELISA experiment in the normal control population near the average value and negative by Western Blot experiment. The second antibody is HRP. Labeled mouse anti-human IgG.
Embodiment 3
[0050] The detection method of this embodiment uses the combined detection of serum markers in the above-mentioned embodiment 1, and the specific steps are as follows:
[0051] 1) Coating: The combined detection serum markers were coated respectively (see Table 1 below for the coating concentration), 100 μL / well, overnight at 4°C.
[0052] 2) Blocking: 2% BSA in PBST (PBS, Tween20) solution, 200 μL / well, overnight at 4°C.
[0053] 3) Washing: wash 3 times with 350 μL / well PBST.
[0054] 4) Primary antibody incubation: the serum to be tested was diluted 1:100 with PBST containing 1% BSA, 100 μL / well, and placed in a semi-water bath at 37°C for 1 hour.
[0055] 5) Washing: wash 5 times with 350 μL / well PBST.
[0056] 6) Secondary antibody incubation: HRP-labeled mouse anti-human IgG was diluted 1:10000 with PBST containing 1% BSA, 100 μL / well, in a semi-water bath at 37°C for 1 hour.
[0057] 7) Washing: wash 5 times with 350 μL / well PBST.
[0058] 8) Color development: TMB ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com