Serum marker capable of evaluating cerebral hemorrhage risk before thrombolysis and application thereof
A marker and serum technology, applied in measurement devices, instruments, scientific instruments, etc., to maintain the natural conformation and activity, overcome surface tension and steric barriers, and facilitate adequate responses
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
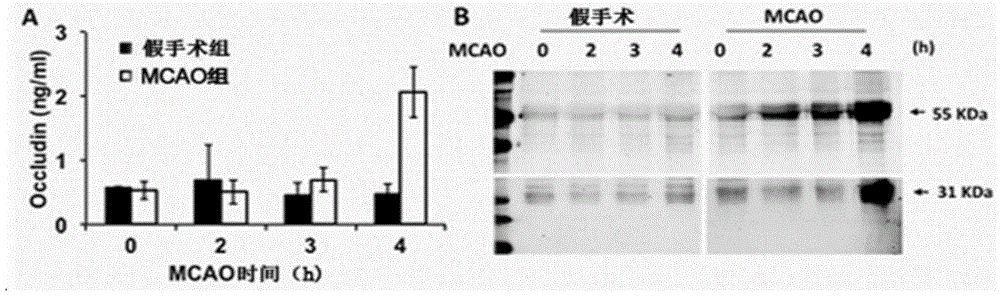
[0054] Serum samples were collected, and the occludin protein concentration in the serum was measured after the occurrence of cerebral ischemia with the basic level as the control. The results were as follows: figure 1 shown. in, figure 1 A shows that the basal level of Occludin protein in serum is low, and after 4 hours of middle cerebral artery embolism (MCAO), the level of Occludin protein fragments in peripheral blood increases significantly compared with the sham operation group; figure 1 In B, the 55kDa protein fragment was increased 2 hours after the middle cerebral artery occlusion (MCAO), and the 31kDa Occludin protein fragment was detected later, and it was not obvious until 4 hours after the middle cerebral artery occlusion (MCAO).
Embodiment 2
[0056] The method for preparing the test kit for detecting the Occludin protein in human serum is as follows:
[0057] Step 1: Preparation of Microsphere Storage Solution
[0058] (1) Activation of microspheres: after the polystyrene microspheres are uniformly dispersed, take 5.0×10 6 Add 100 μl washing buffer (PBS, 0.05% Tween) to the microspheres, shake at high speed for 15 seconds, suspend the microspheres with 80 μL activation buffer after washing, add 10 μL carbodiimide (EDC, 50 mg / ml), and quickly add 10 μL Sulfo-NHS (50mg / ml), shake at high speed for 30s, shake at room temperature for 20min in the dark, then wash twice with 500μL 0.01MpH7.4PBS, centrifuge at 12000r / min for 3min each time, discard the supernatant, and finally suspend the microspheres with 100μL0.01MpH7.4PBS .
[0059] (2) Cross-linking of microspheres: Add 100 μg of anti-Occludin protein monoclonal antibody to the activated microspheres, dilute to 500 μL with 0.01M pH7.4 PBS, wrap in tinfoil, shake at ...
Embodiment 3
[0080] 1. the test kit of embodiment 2, by 1 piece of 96 hole filter plate, 2 bottles of standard substance, 1 bottle of sample diluent, the polystyrene microsphere that the surface has been coupled with anti-GFAP antibody, 1 bottle of microsphere storage solution , 1 bottle of enzyme conjugate, 1 bottle of substrate A1 bottle, 1 bottle of substrate B1, 20× washing solution, 20 self-adhesive coverslips and 1 instruction manual.
[0081] 2. the application of the test kit of embodiment 2
[0082] (1) Dilution of the standard substance: Dilute each bottle to 1mL with the sample diluent before use. After standing for 10 minutes, mix it upside down repeatedly. mL, 12.5ng / mL, 3.12ng / mL, 0.78ng / mL, 0.05ng / mL, the sample diluent is 0ng / mL.
[0083] (2) Adding samples: Shake and mix the microspheres at a medium speed before use, add 10 μL microspheres containing about 50,000 Occludin protein monoclonal antibodies to each well, vacuum filter and wash twice with 100 μL washing liquid, an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com