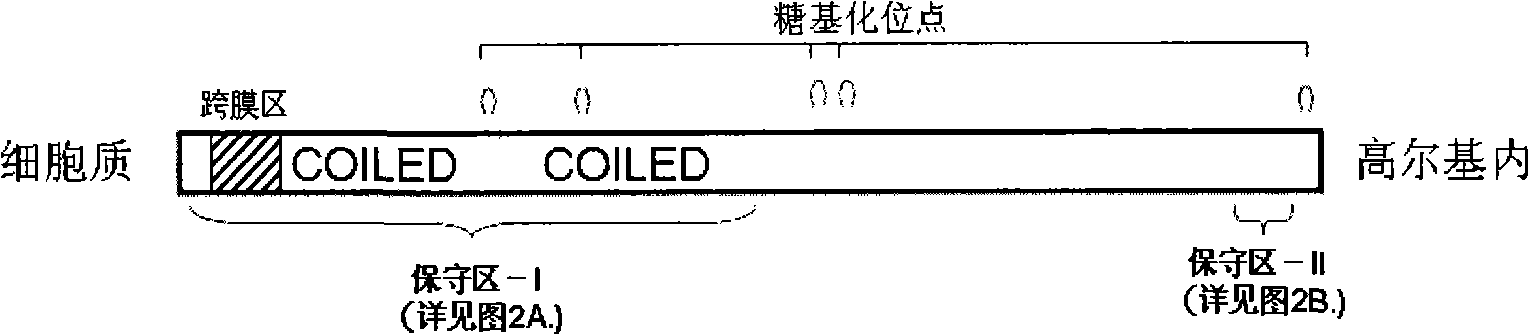
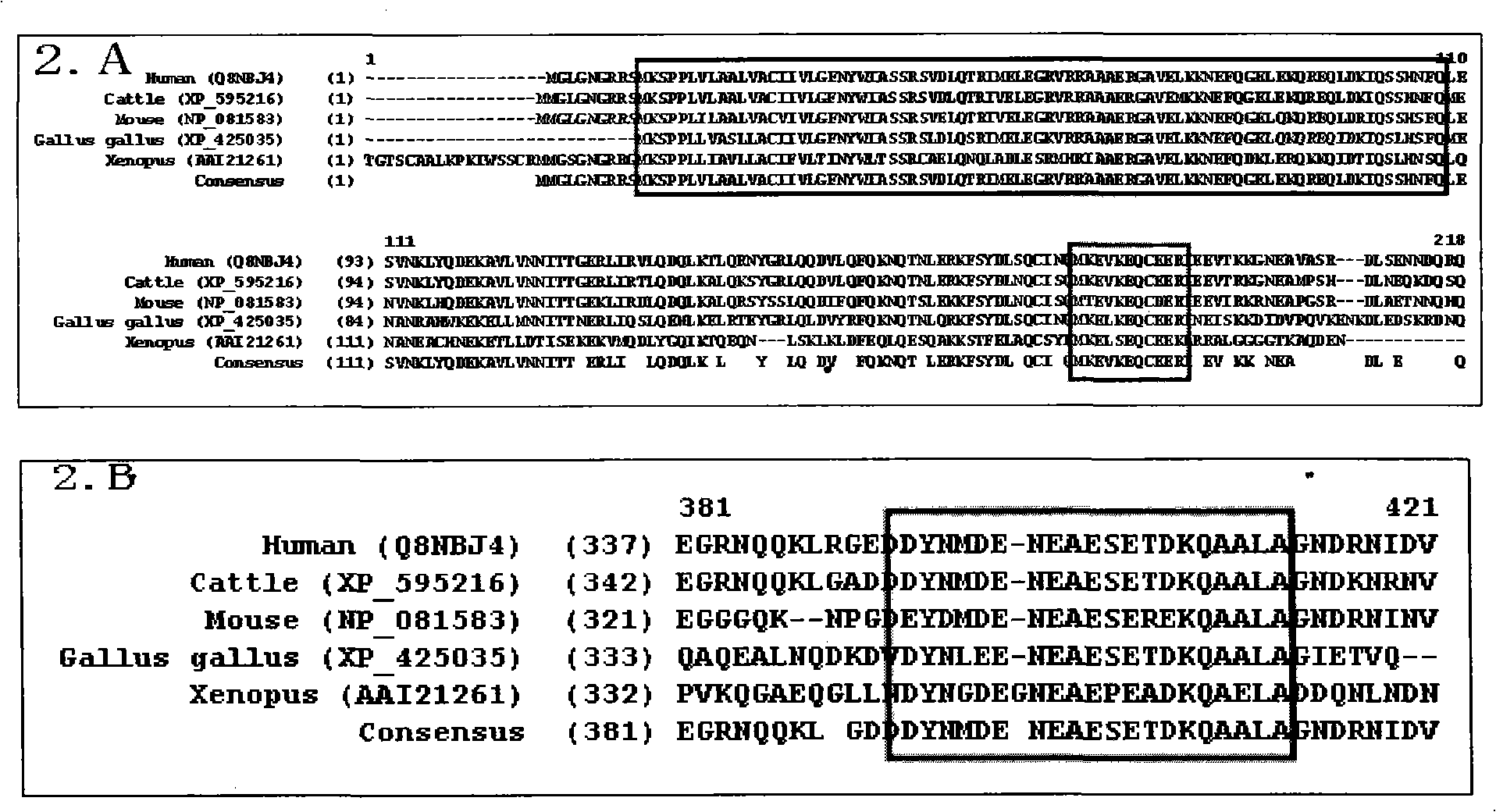
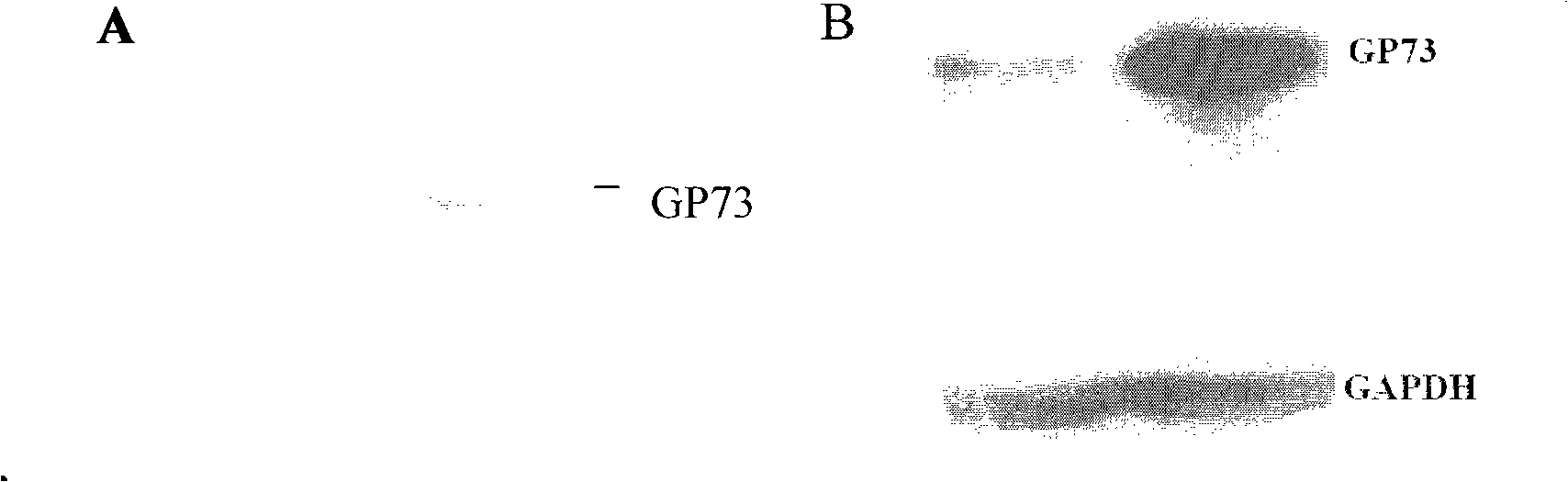
Liver disease blood serum specific protein and use thereof
A liver disease, serum protein technology, applied in the direction of serum albumin, albumin peptides, animal/human proteins, etc., can solve the problems of high sensitivity and specificity, cumbersome operation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Preparation of gp73 recombinant protein
[0060] (1) Cloning the gp73cDNA sequence onto the expression vector:
[0061] Primer design: Add BamH I and Hind III restriction sites to both ends of the sense and antisense primers respectively. The primer sequences are as follows:
[0062] 5'-CATAGGGATCCCTCCAGACACGGATCATGGAGCTGGAAGGC-3'
[0063] 5'-GAGAGAAGCTTTCAGAGTGTATGATTCCGCTTTTCACGCTG-3'.
[0064] The gp73cDNA sequence was amplified by reverse transcription using mRNA as a template.
[0065] Expression vector: pTrichisA (there is a 6×his tag upstream of the BamH I restriction site of the vector)
[0066] The vector and the target fragment were double-digested with BamH I and Hind III respectively, and the digested products were ligated by T4 ligase, and transformed into TOP10 competent (purchased from TAKARA company, the transformation step was operated according to the competent instructions), and ampicillin plate 37 Cultivate overnight at ℃, pick a single clone, id...
Embodiment 2
[0070] Preparation of gp73 antibody
[0071] (1) The steps of gp73 monoclonal antibody preparation are:
[0072] Animal immunization procedure: Balb / c mice were used as immunized animals, and the immunization dose was 50-100ug / mL. At the first immunization, the immunogen was mixed with an equal amount of Freund's complete adjuvant to make an emulsifier, and injected intraperitoneally, with an interval of 2 weeks Afterwards, the same amount of immunogen was emulsified with the same amount of Freund's incomplete adjuvant, and after an interval of 10 days, the antigen was directly immunized. After an interval of one week, the spleen or tail vein was boosted once, and the spleen cells were collected 3 days later.
[0073] Cell fusion and cloning: Splenocytes from immunized mice were fused with SP2 / 0 myeloma cells at a ratio of 5-10:1, cell supernatants were measured by indirect ELISA, positive wells were screened, and antibodies were identified by westblot , using the limiting di...
Embodiment 3
[0079] The construction of gp73 detection kit:
[0080] Set up the gp73 ELISA kit to include the following components:
[0081] (1) Enzyme plate coated with gp73 monoclonal antibody;
[0082] (2) Enzyme-labeled rabbit anti-gp73 antibody;
[0083] (3) gp73 standard solution;
[0084] (4) Substrate chromogenic solution and stop solution;
[0085] (5) Concentrate the washing solution.
[0086] The preparation steps of the enzyme label plate coated with gp73 monoclonal antibody in the kit of the present invention are:
[0087](1) Dilute the gp73 monoclonal antibody to 1ug / mL with coating buffer;
[0088] (2) Add 100 μl of diluted monoclonal antibody to each well of the 96-well ELISA plate, place at 4°C for 18 hours after sealing, pour off the coating solution, wash 3 times with washing solution, and pat dry;
[0089] (3) Add 200 μl of blocking solution to each well of the 96-well ELISA plate, incubate at 37° C. for 2 hours, pour off the liquid in the well, wash and drain, an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com