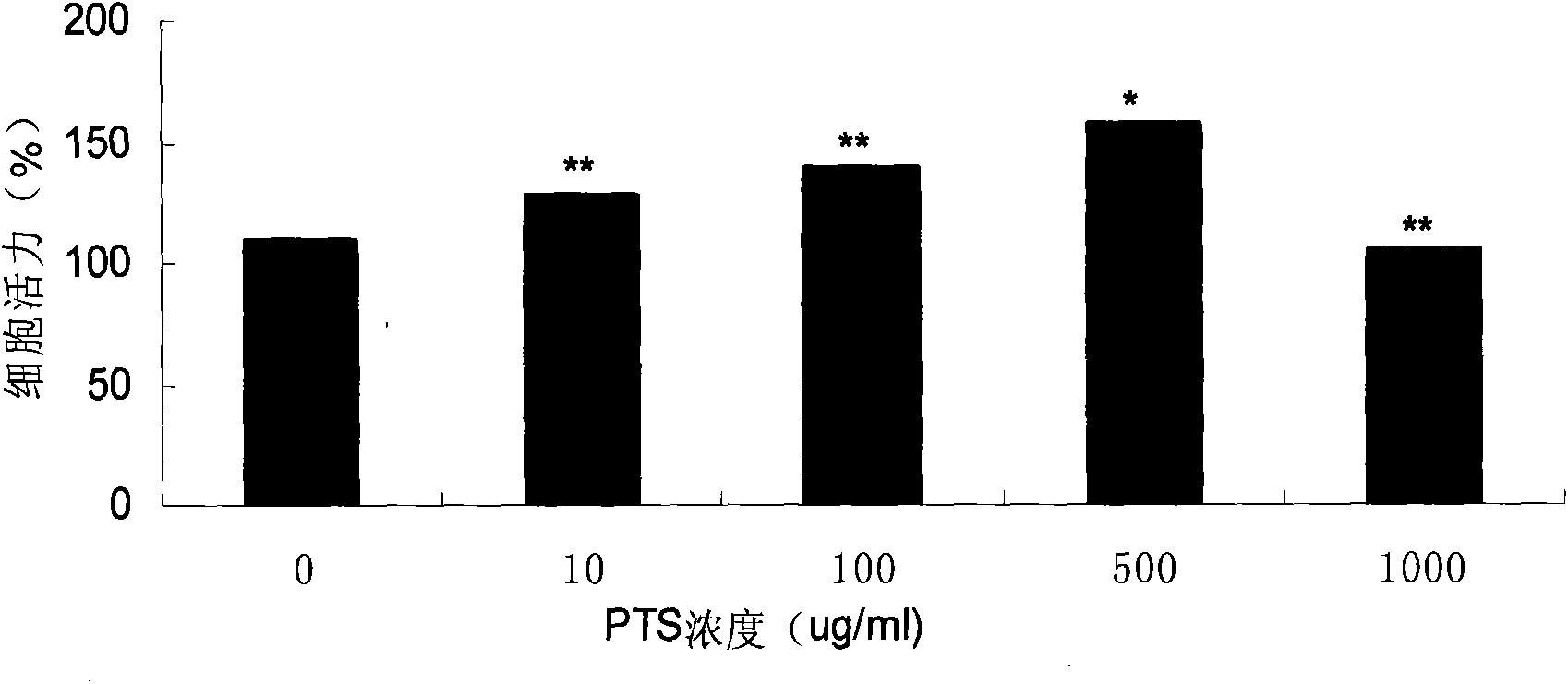
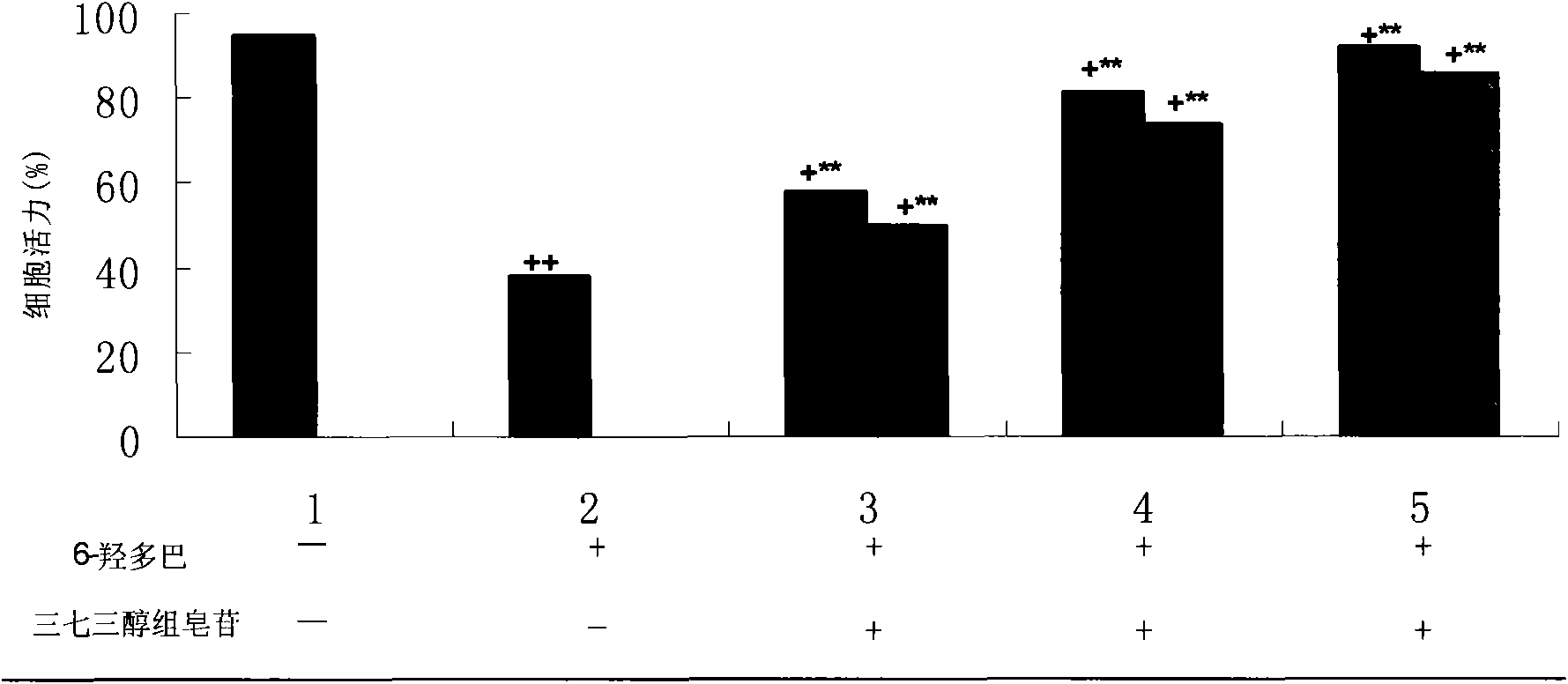
Medicinal composition for treating Parkinson disease
A technology of Parkinson's disease and composition, which is applied in the direction of drug combination, medical formula, nervous system diseases, etc., can solve the problems of ineffective prevention and treatment of rapid development of PD, large toxic and side effects, and inaccurate curative effect, so as to improve blood flow in the brain , quality controllable, and the effect of improving the quality of life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Embodiment 1: the preparation of composition
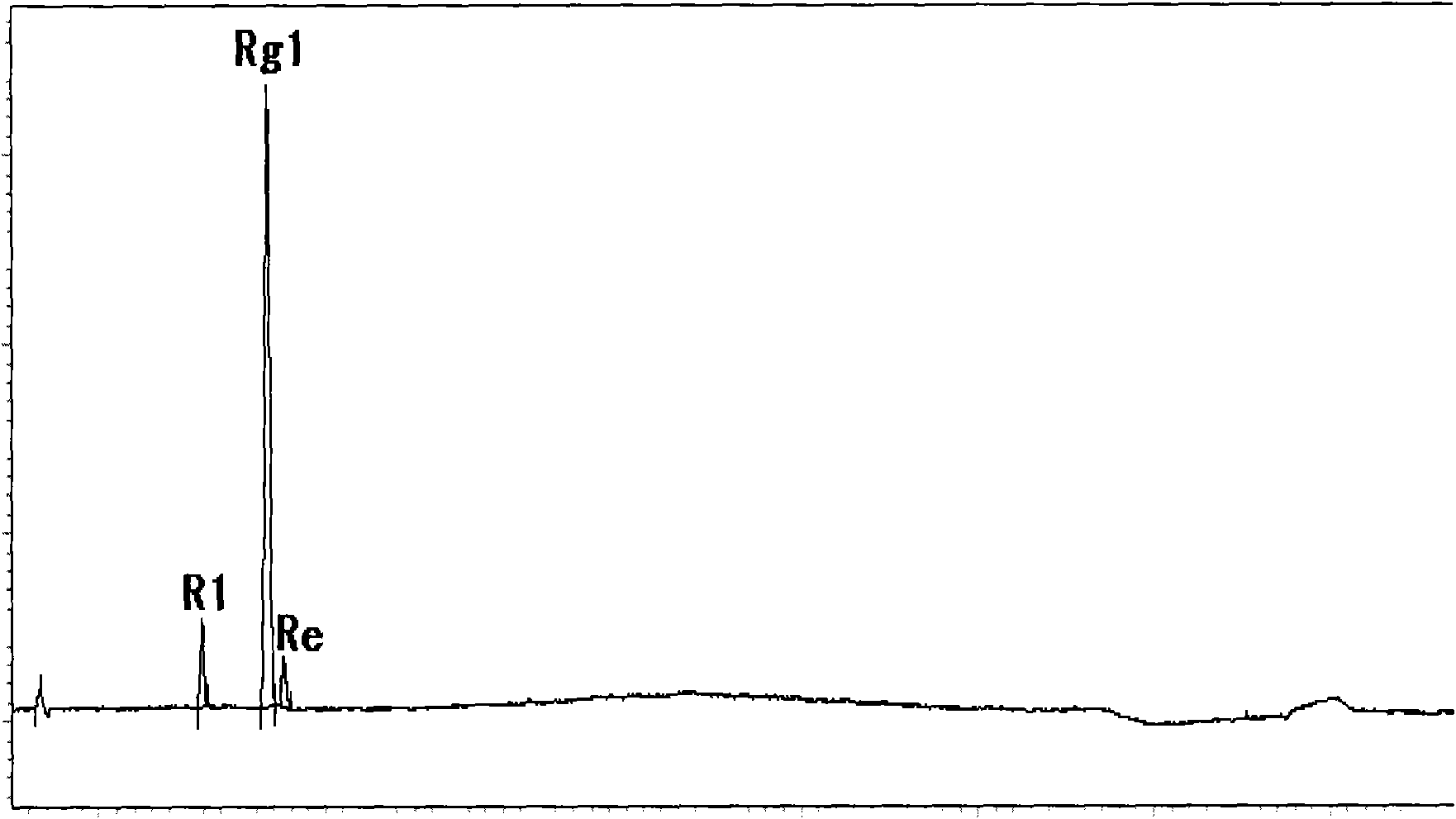
[0037] Take 50 g of total saponins of Panax notoginseng, add appropriate amount of water to heat to completely dissolve, the obtained aqueous solution is loaded onto a macroporous adsorption resin column D101 type and eluted with 30% ethanol, the eluate is collected, and concentrated under reduced pressure to complete dryness to obtain Panax notoginseng Triol group saponin 1#.
Embodiment 2
[0039] Take 50 g of total saponins of Panax notoginseng, add appropriate amount of water and heat to completely dissolve, and the obtained aqueous solution is loaded onto a macroporous adsorption resin column D101 and eluted with 40% ethanol, the eluate is collected, concentrated under reduced pressure to complete dryness, and Panax notoginseng is obtained Triol group saponin 2#.
Embodiment 3
[0041]Take 50g of Panax notoginseng total saponins, add appropriate amount of water and heat to completely dissolve, the obtained aqueous solution is loaded onto a macroporous adsorption resin column and eluted with 50% ethanol, the eluate is collected, concentrated under reduced pressure to complete dryness, and notoginseng triol is obtained Group saponin 3#.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com