Traditional Chinese medicine composition of bone healing medicine, preparing method thereof and detecting method thereof
A detection method and composition technology, applied in the field of traditional Chinese medicine, can solve problems such as low accuracy and sensitivity of thin-layer chromatography, affecting application, unstable types and contents of active ingredients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] The preparation of embodiment 1 Guyuling capsule
[0054] The prescription and preparation method are as follows:
[0055] Prescription (1000 capsules): 60g Panax notoginseng; 60g dried blood; 30g safflower; 20g frankincense (made); 20g rhubarb; 20g angelica; 20g Chuanxiong; 20g myrrh (made); 20g; Rhizoma Drynaria 20g; Dipsacus 20g; Natural Copper (calcined) 20g; Wujiapi 20g and borax 20g.
[0056] The above sixteen flavors are mixed and pulverized into fine powder, dried, mixed evenly, packed into capsules, and obtained.
Embodiment 2
[0057] Notoginsenoside R in Example 2 Guyuling Capsules 1 , Ginsenoside Rg 1 and ginsenoside Rb1 content detection
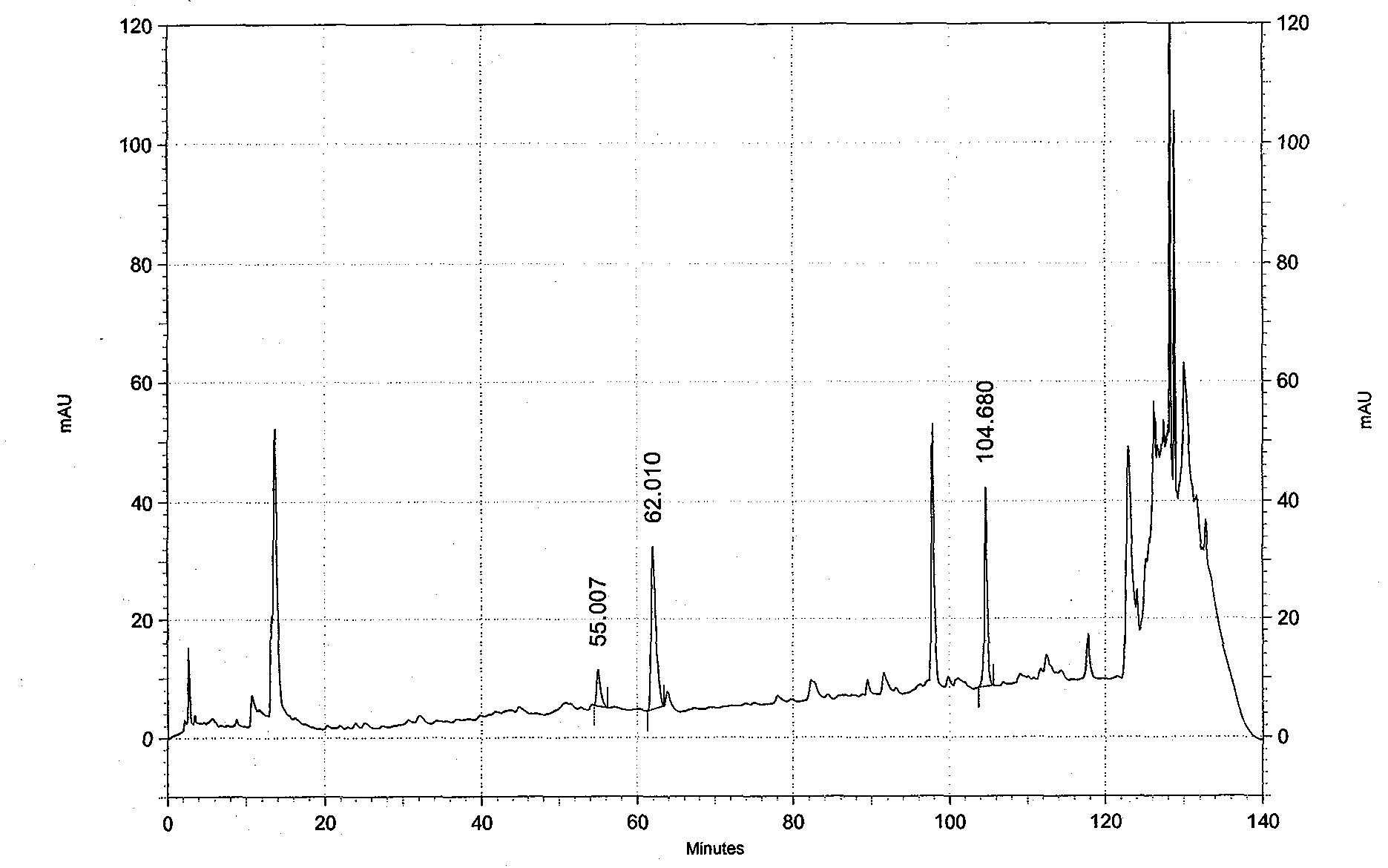
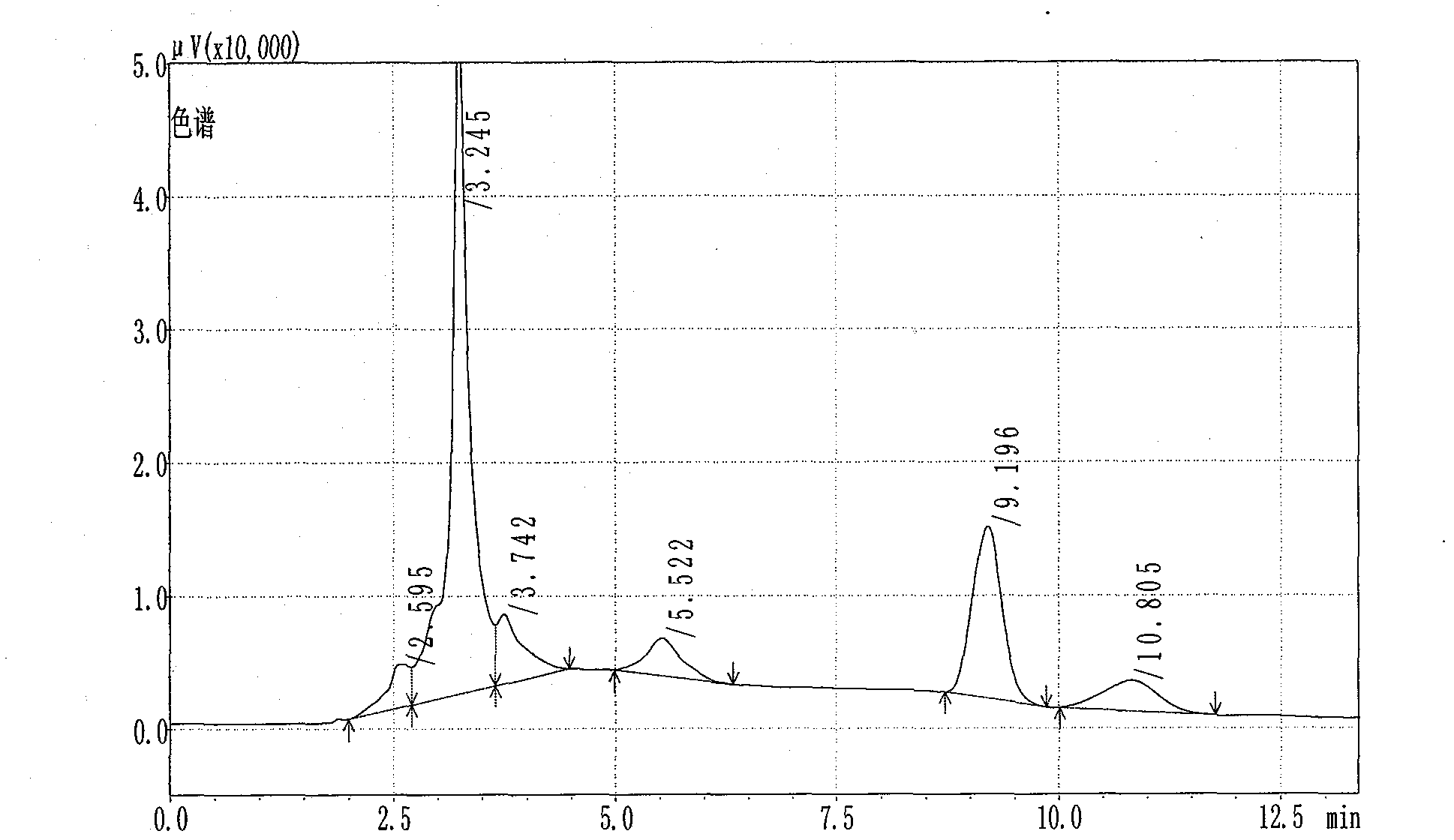
[0058] In this example, the notoginsenoside R in the Guyuling capsules prepared in Example 1 was detected by high performance liquid chromatography. 1 , Ginsenoside Rg 1 and ginsenoside Rb 1 content.
[0059] Instrument: high performance liquid chromatography, Alltech, USA, model UVIS-201, UV detector.
[0060] Reagents: Acetonitrile is chromatographically pure reagent; water is ultrapure water; other reagents are analytically pure.
[0061] Reference substance: Ginsenoside Rb 1 (110704-200318), Ginsenoside Rg 1 (110703-200424) and notoginsenoside R 1 (110745-200414) were purchased from China Institute for the Control of Pharmaceutical and Biological Products.
[0062] The corresponding instruments, reagents and reference substances involved in each of the following examples are the same.
[0063] 1. Chromatographic conditions
[0064] Chromatographic...
Embodiment 3
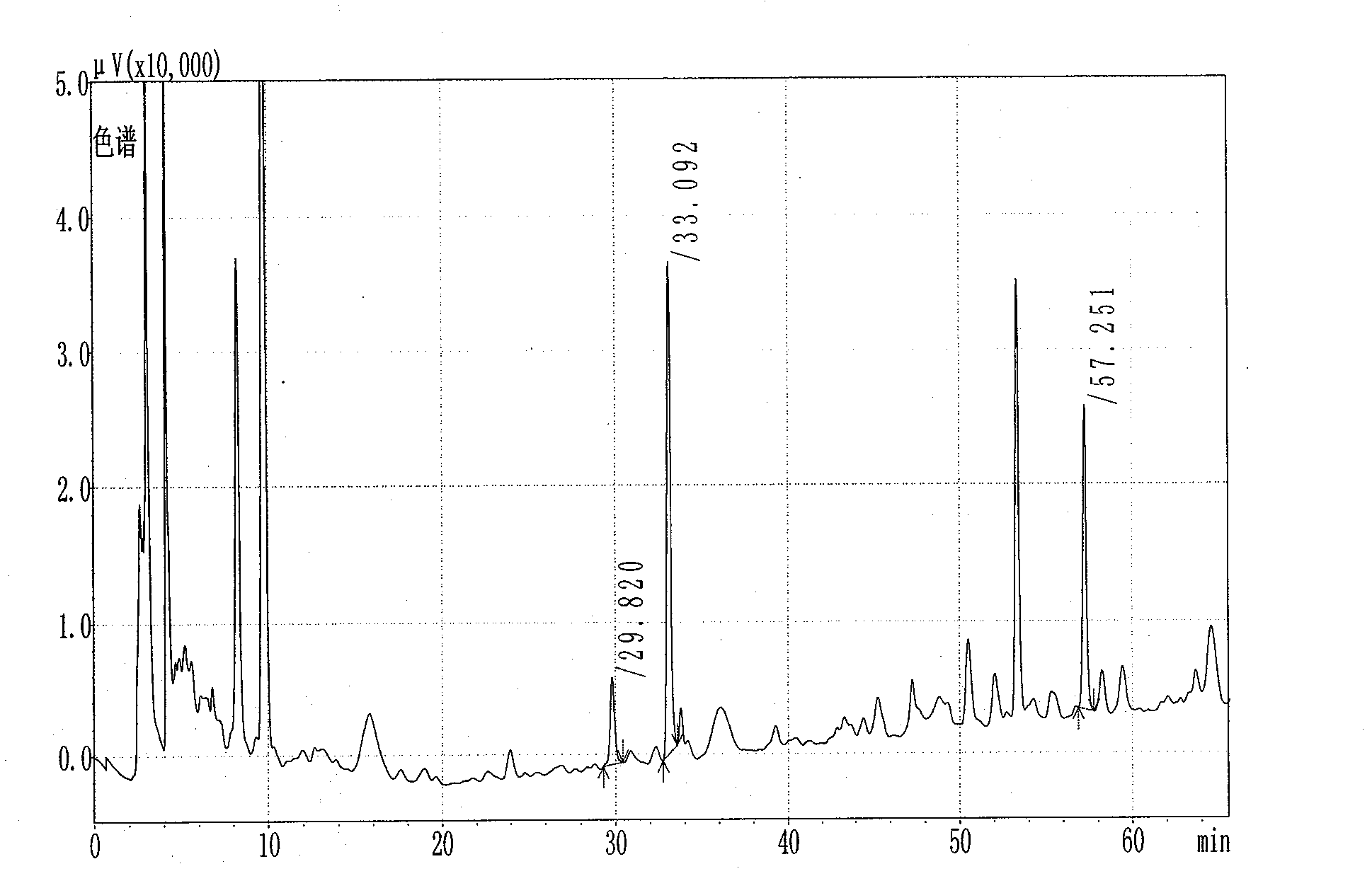
[0097] The detection of the content of hydroxysafflor yellow A in embodiment 3 Guyuling capsules
[0098] In this example, the content of hydroxysafflower yellow A in the Guyuling capsules prepared in Example 1 was detected by high performance liquid chromatography.
[0099] 1. Chromatographic conditions:
[0100] Chromatographic column: C18 chromatographic analysis column (4.6mm×250mm, 5μm), guard column (ODS, 4.0mm×3.0mm); mobile phase: methanol-acetonitrile-0.7% (volume ratio) phosphoric acid aqueous solution (volume ratio=62:2 : 72); UV detection wavelength: 403nm; flow rate: 1.0ml / min; column temperature: 30C.
[0101] Under the above chromatographic conditions, the separation between the reference product hydroxysafflor yellow A and the adjacent chromatographic peak is greater than 1.5, and the number of theoretical plates is not less than 1500 according to the peak of hydroxy safflower yellow A.
[0102] 2. Preparation of reference solution
[0103] Accurately weigh ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com