Method for preparing notoginsenoside R1 and ginsenoside Rg1, Re, Rb1 and Rd
A technology of notoginseng saponins and ginsenosides, which is applied in the fields of steroids and organic chemistry, which can solve the problems of prolonged analysis time and unfavorable separation and preparation of saponins, and achieve the effects of simple process, low cost and reduced production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
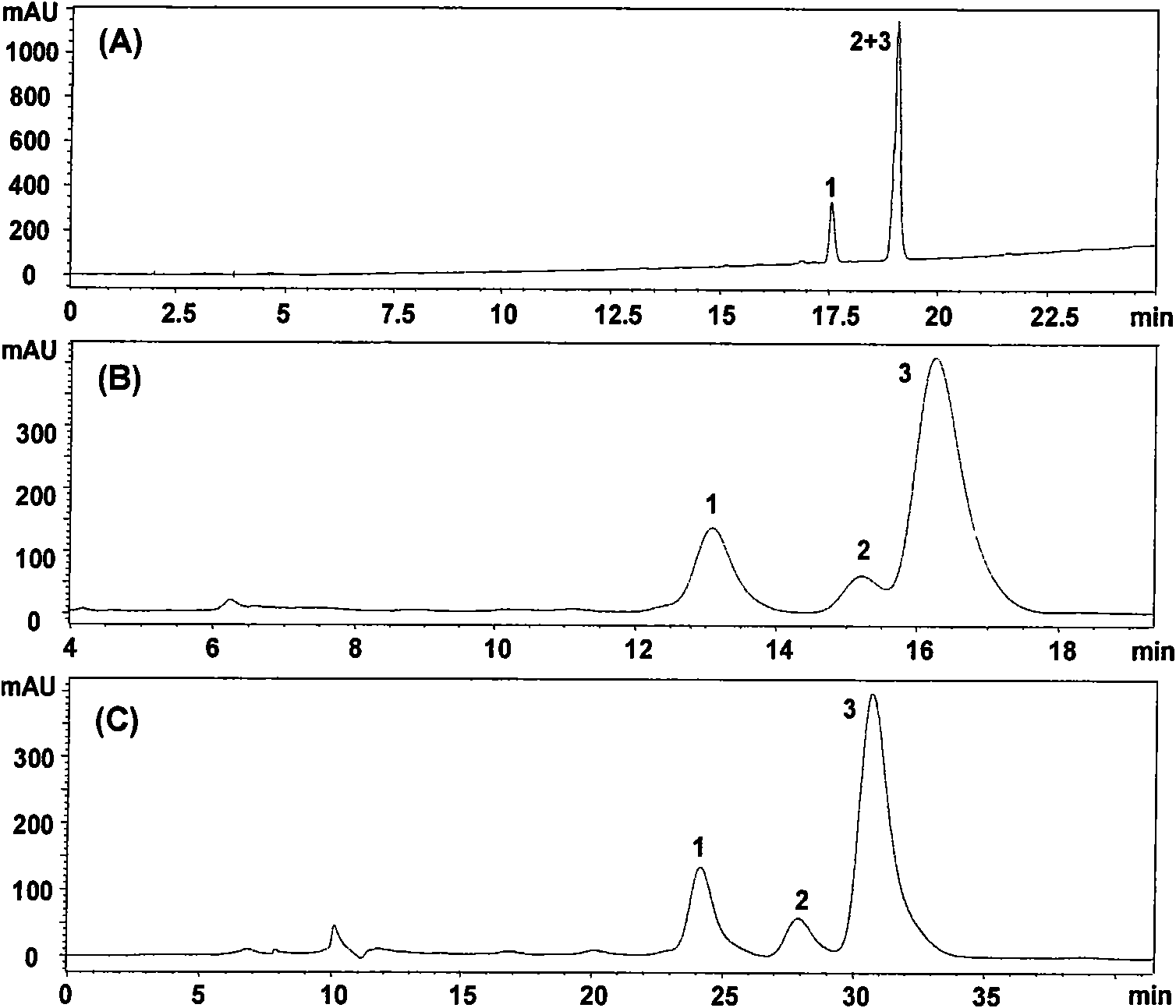
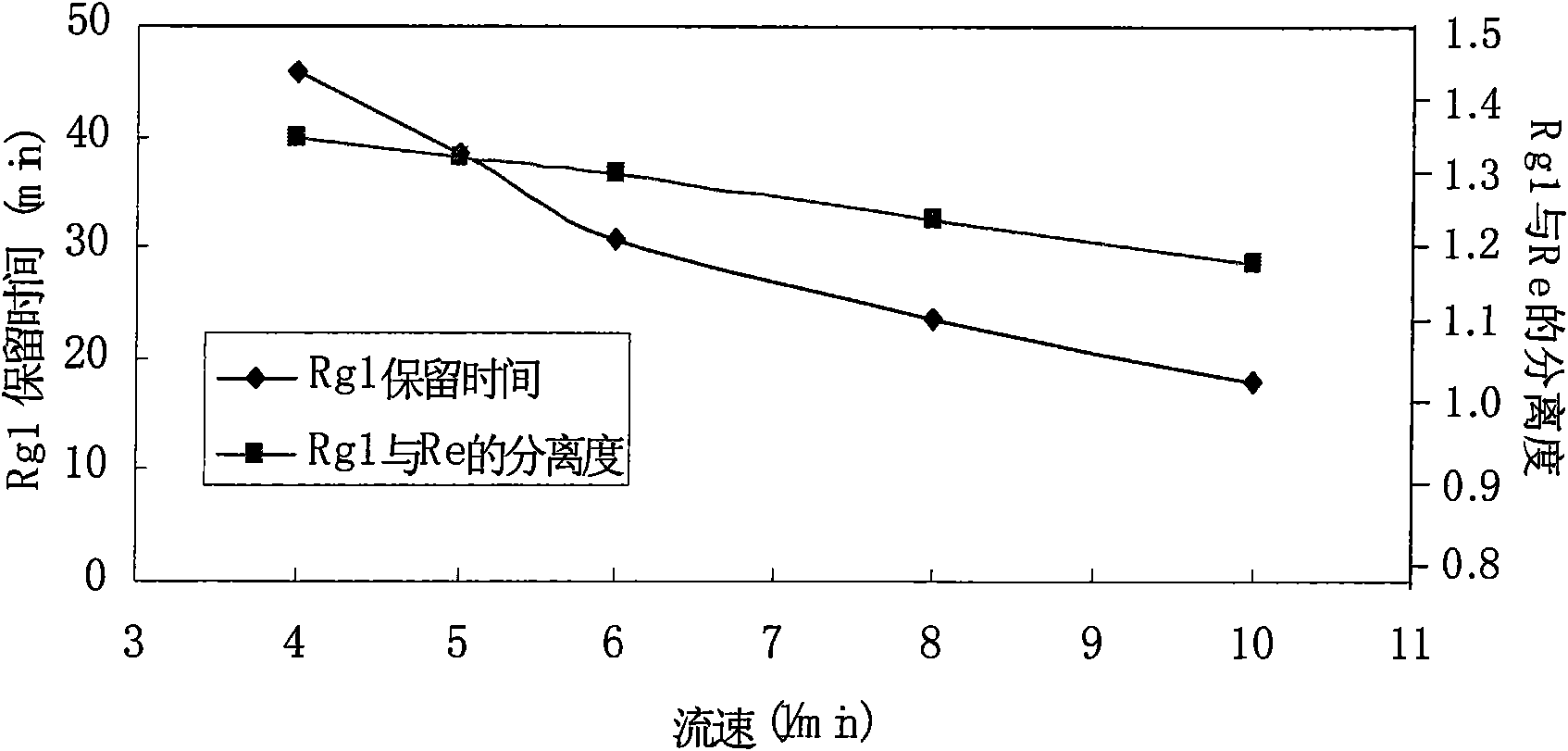
[0035] 200g of notoginseng medicinal material powder, ultrasonically extracted with 1000ml of 70% ethanol for 2h, repeated three times to combine the extract, and evaporated the solvent in vacuo to obtain the extract. The above extract was fully dissolved in 200ml of 40% (v / v) ethanol-water. The solution of the above-mentioned extract was applied to preparative reversed-phase high-performance liquid chromatography, with Alltima C 18 (250 × 22mm, 10 μ m) as the chromatographic column, 203nm detection, flow rate 6.0ml / min, 40% (v / v) ethanol- The water system (A phase) and 60% (v / v) ethanol-water system (B phase) were used as mobile phases for gradient elution, and the elution procedures were shown in Table 2. Notoginsenoside R1, ginsenoside Re, and Elution peaks of Rg1, Rb1 and Rd.
[0036] Table 2 Gradient elution program
[0037] time (min)
[0038] The collected liquids of ginsenosides were combined, the solvent was evaporated under reduced pressure, and vacuum fr...
Embodiment 2
[0040] Take 20 g of commercially available Panax notoginseng saponins, fully dissolve in 200 ml of pure water, apply the solution of the above extract to preparative reversed-phase high-performance liquid chromatography, use Alltima C18 (250 × 22 mm, 10 μm) as the stationary phase, 203 nm Detection, flow rate 6.0ml / min, 40% (v / v) ethanol-water system (A phase) and 60% (v / v) ethanol-water system (B phase) as mobile phase for gradient elution, elution program As shown in Table 2, the elution peaks of notoginsenoside R1, ginsenoside Re, Rg1, Rb1 and Rd were collected respectively (see Figure 5 ).
[0041] The collected liquids of ginsenosides were combined, the solvent was evaporated under reduced pressure, and vacuum freeze-dried to obtain freeze-dried powders of notoginsenoside R1, ginsenoside Rg1, Re, Rb1 and Rd monomer compounds.
Embodiment 3
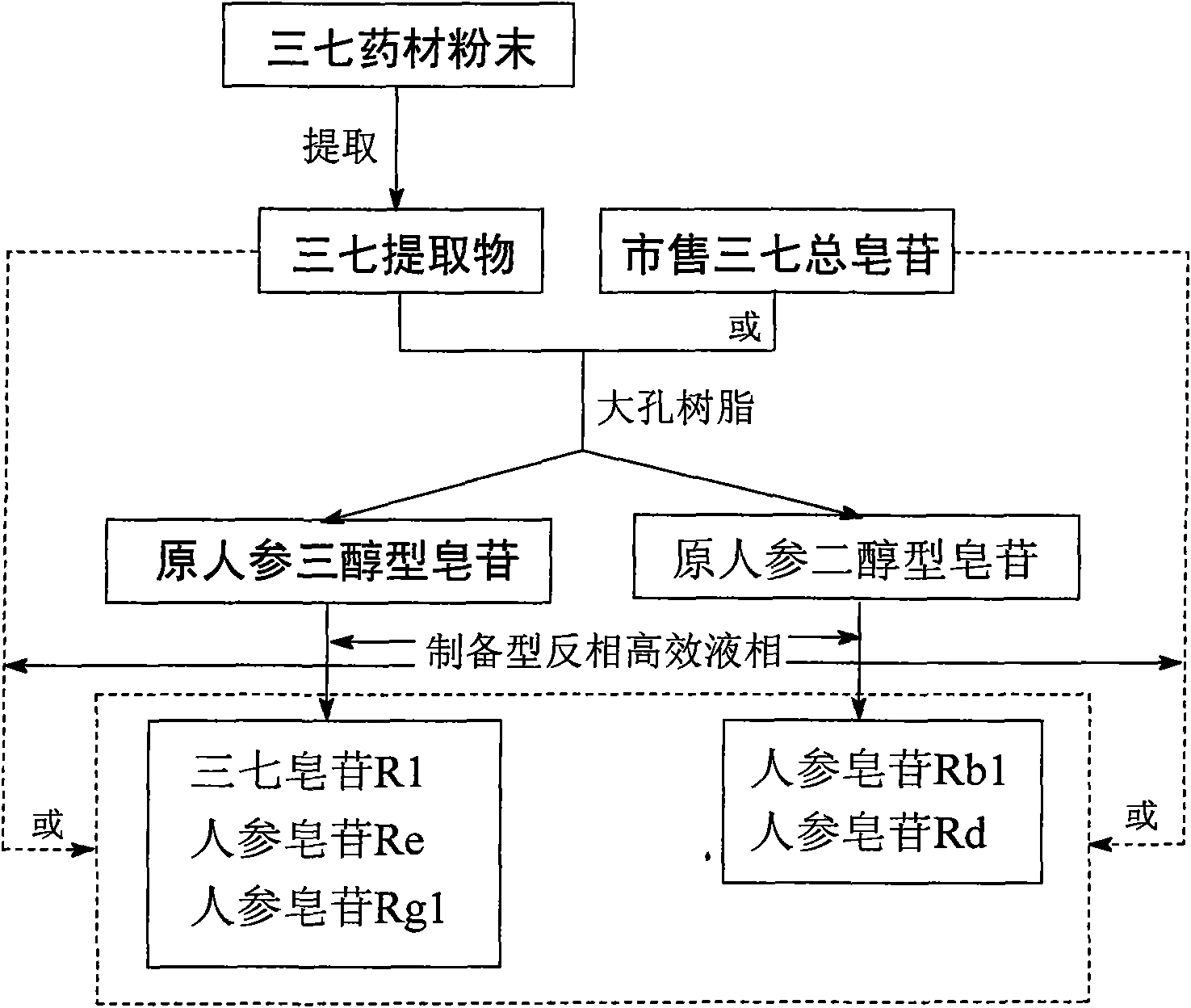
[0043] 200g of notoginseng medicinal material powder, ultrasonically extracted with 1000ml of 70% ethanol for 2h, repeated three times to combine the extract, and evaporated the solvent in vacuo to obtain the extract. The above-mentioned extract is fully dissolved in 200ml water, applied to 500g D-101 macroporous adsorption resin, first eluted with 3000ml of pure water, then eluted with 30% (v / v) ethanol-water 6000ml, and finally washed with 3000ml 80% (v / v) ethanol-water is carried out eluting and combining respectively 30% and 80% ethanol-water eluate, evaporates solvent under reduced pressure, vacuum freeze-drying, obtain intermediate notoginseng protopanaxatriol type saponin and Protopanaxadiol saponins.
[0044] The protopanaxatriol-type saponins obtained by the above separation were applied to preparative reversed-phase high-performance liquid chromatography, with Alltima C18 (250 × 22mm, 10 μm) as the stationary phase, and 40% (v / v) ethanol-water system as the mobile ph...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com