Composition of traditional Chinese medicine effective constituent for preventing and treating diseased associated with cerebral ischemia injury
A technology of active ingredients and compositions, applied in the field of compositions for the prevention and treatment of cerebral ischemic injury, can solve problems such as the inability to truly control product quality, affect the stability of clinical efficacy, and difficult effective control of product quality, so as to protect ischemic injury , prevent platelet aggregation, reduce the effect of cerebrovascular resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0146] Embodiment 1, tablet
[0147] raw material:
[0148] Ginsenoside Rb 1 (content≥95%), ginsenoside Rd (content≥95%), ginsenoside Re (content≥95%), ginsenoside Rg 1 (content ≥ 95%); the above four ginsenosides were purchased from Jilin Hongjiu Biotechnology Co., Ltd.
[0149] Stilbene glycosides (content ≥ 70%), provided by Tianjin Huilv Technology Co., Ltd.
[0150] Ginkgolides (total lactone content ≥ 95%; ginkgolide B ≥ 40%), provided by Shanghai Autuo Information Technology Co., Ltd.
[0151] Quercetin and kaempferol (content ≥ 95%) were purchased from Shaanxi Huike Plant Development Co., Ltd.
[0152] Ginsenoside Rb 1 10kg, Ginsenoside Rd 1.0kg, Ginsenoside Re 4kg, Ginsenoside Rg 1 10kg, stilbene glycosides 8kg, ginkgolides 4kg, flavonoid mixture 3kg (quercetin 1.5Kg plus kaempferol 1.5Kg), mixed according to equal increment method to make active ingredient mixture (FSWXN5) 40Kg, add starch 25kg, 35 kg of dextrin, mixed evenly, granulated with 75% alcohol, dr...
Embodiment 2
[0155] Embodiment 2, capsule
[0156] Raw material source is with embodiment 1
[0157] Ginsenoside Rb 1 4kg, Ginsenoside Rd 4.0kg, Ginsenoside Re 5kg, Ginsenoside Rg 1 6kg, 2.0kg of stilbene glycosides, 5kg of ginkgolides, 2kg of flavonoid mixture, mixed according to the method of equal increase to make active ingredient mixture (FSWXN2) 28Kg, add 12kg of starch, 10kg of dextrin, mix well, and make Capsule powder 50kg packs No. 3 capsules, and a total of 333,300 capsules are prepared, each 0.15g, containing FSWXN 84mg / grain.
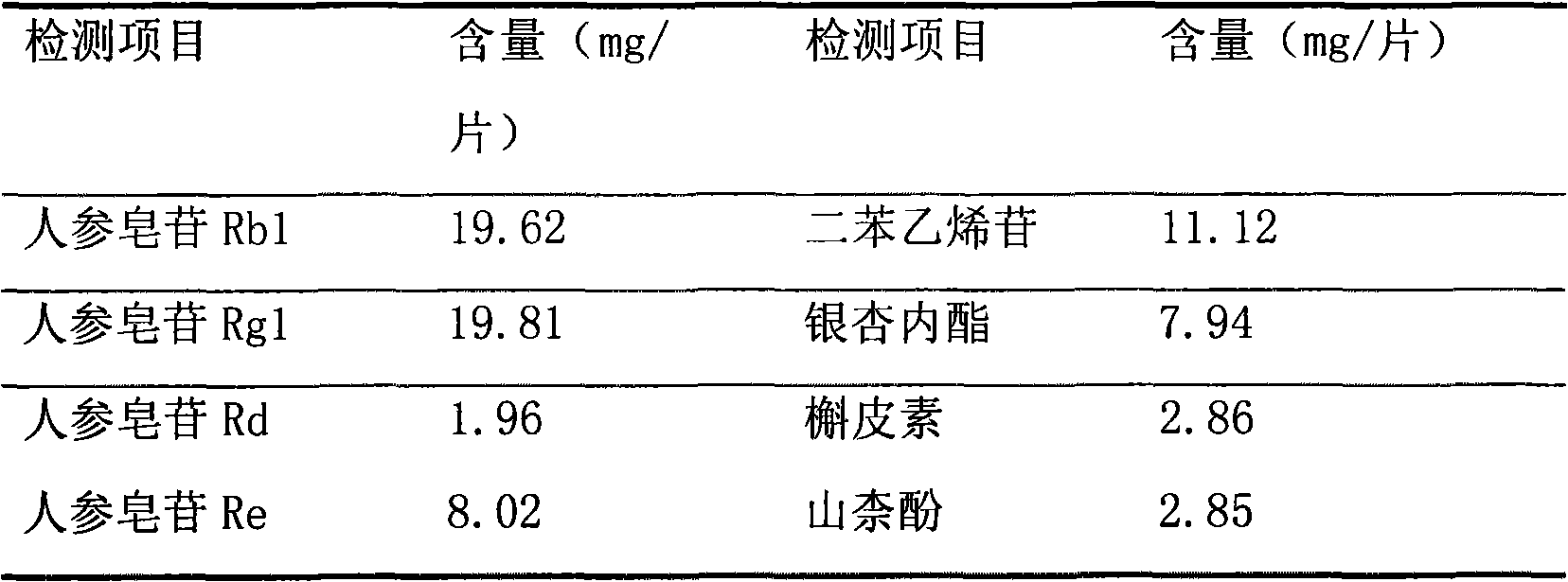
[0158] The content of each active ingredient in the compound Shenwu Xingnao active ingredient (SWXNF2) capsule was determined by HPLC method, and the results are shown in the following table.
[0159]
[0160] Compound Shenwu Xingnao active ingredient composition 2 (FSWXN2) capsule powder obtained by the present invention, its each active ingredient content measured by HPLC method is: ginsenoside Rb 1 7.62%, Ginsenoside Rg 1 11.38%, ginsenos...
Embodiment 3
[0161] Embodiment 3 honey pill
[0162] Raw material source is with embodiment 1
[0163] Ginsenoside Rb 1 2.0kg, Ginsenoside Rd 2.0kg, Ginsenoside Re 3.0kg, Ginsenoside Rg 1 8.0kg, stilbene glycosides 10.0kg, ginkgolides 2kg, flavonoid mixture 2kg, mixed according to the method of equal increase to make compound ginseng root refreshing active ingredient composition 1 (FSWXN1) 29.0kg, add starch 20.5kg, paste Refined 15.kg, mixed evenly, added honey 10kg to make small honeyed pills, dried at 60°C, made into 72.5kg dry pills, filled with No. 0 capsules, and made a total of 241,000 capsules, each 0.30g, containing FSWXN 1 120mg / capsule.
[0164] Oral dosage: 1 capsule each time, 2 times a day.
[0165] The results of the content of each active ingredient in the small honeyed pills in each sachet of capsules are determined in the following table by HPLC method.
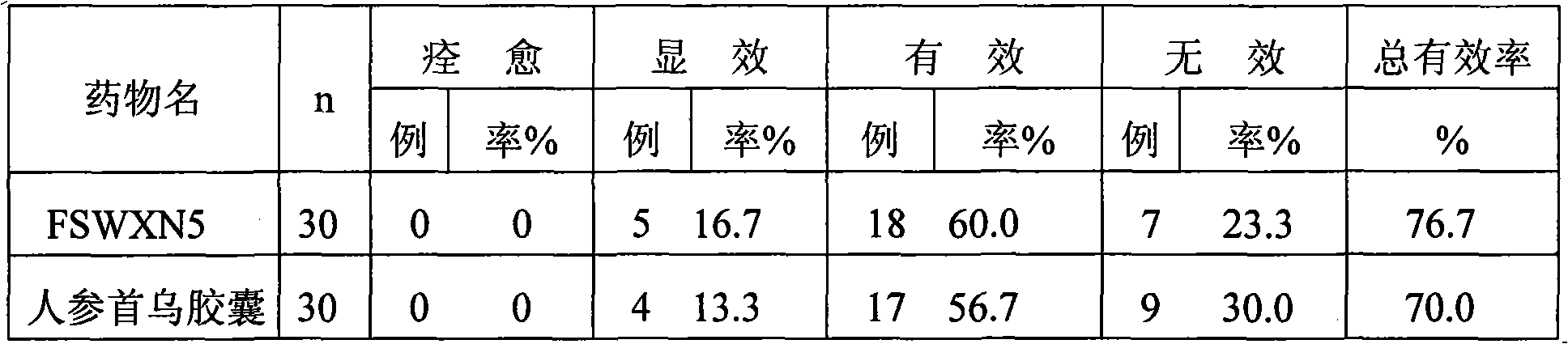
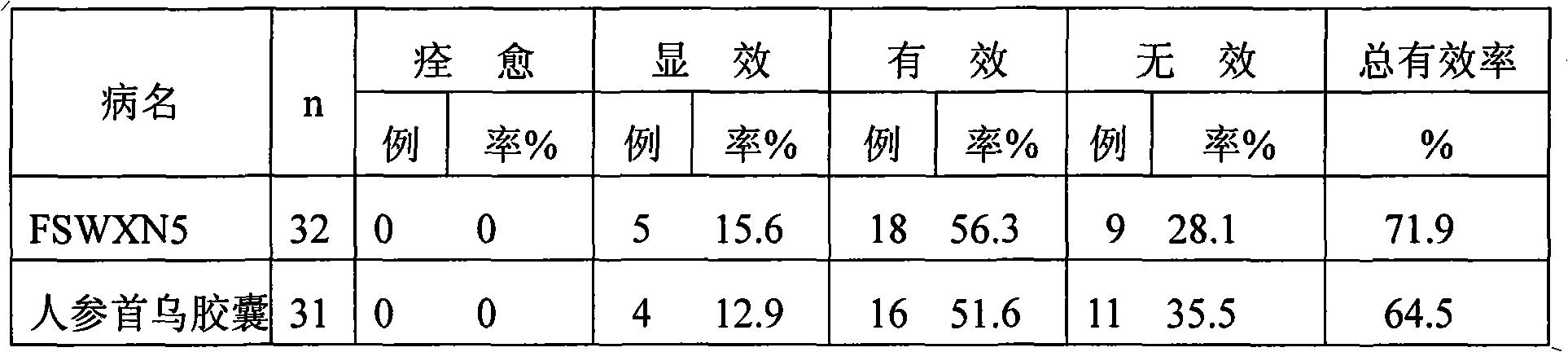
[0166] Trial for the clinical treatment of stroke sequelae, 2 times a day, 1 capsule each time, for 60 days,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com